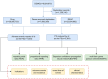
Safety evaluation of baloxavir marboxil: analysis and discussion utilizing real adverse events from the FAERS database
- PMID: 40420187
- PMCID: PMC12107739
- DOI: 10.1186/s40360-025-00940-0
Safety evaluation of baloxavir marboxil: analysis and discussion utilizing real adverse events from the FAERS database
Abstract
Background: As a novel anti-influenza agent, baloxavir marboxil lacks real-world safety data in large populations. Therefore, this study aimed to investigate adverse drug events (ADEs) associated with baloxavir marboxil by analyzing the Food and Drug Administration Adverse Event Reporting System (FAERS) database.
Methods: Adverse event reports involving baloxavir marboxil were extracted from the FAERS database spanning the fourth quarter of 2018 to the third quarter of 2023. Demographic characteristics and reporter profiles were analyzed to characterize the exposed population. A disproportionality analysis was performed using four validated pharmacovigilance algorithms: reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and multi-item gamma Poisson shrinker (MGPS). These complementary approaches were employed to detect, prioritize, and validate potential safety signals.
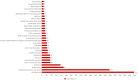
Results: Analysis of 8,824,675 ADE reports from the FAERS database identified 1,654 cases (0.19%) associated with baloxavir marboxil. Pediatric patients (< 18 years) exhibited the highest ADE reporting rate. Geospatial analysis revealed marked clustering, with 98.97% of reports originating from the United States (63.2%) and Japan (35.77%). We detected 47 significant safety signals spanning 27 System Organ Classes (SOCs), including established reactions such as pneumonia (n = 90) and vomiting (n = 77). Novel signals emerging from the analysis comprised hemorrhagic diathesis (n = 3), rhabdomyolysis (n = 25), hepatic dysfunction (n = 13), and cardiorespiratory arrest (n = 7). Notably, bleeding-related events (e.g., ischemic colitis, IC025 = 5.03) and neurological complications (e.g., febrile delirium, IC025 = 9.12) demonstrated statistically significant associations.
Conclusion: This pharmacovigilance study identifies previously undercharacterized safety signals associated with baloxavir marboxil, including hemorrhagic complications, liver dysfunction, rhabdomyolysis, and life-threatening cardiorespiratory events. Pediatric populations and patients on anticoagulants may require heightened monitoring. While these findings provide critical pharmacovigilance insights, our study is inherently constrained by the spontaneous reporting system, which introduces potential underreporting, reporting biases, and confounding factors. Future research could employ more rigorous prospective study designs, integrating clinical trials and epidemiological studies, to more accurately assess the safety risks of baloxavir marboxil.
Keywords: Adverse drug reactions; Baloxavir marboxil; FAERS database; Influenza; Pharmacovigilance; Signal mining analysis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. This study was deemed non-human subject related research. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Adverse events associated with oseltamivir and baloxavir marboxil in against influenza virus therapy: A pharmacovigilance study using the FAERS database.PLoS One. 2024 Nov 13;19(11):e0308998. doi: 10.1371/journal.pone.0308998. eCollection 2024. PLoS One. 2024. PMID: 39536015 Free PMC article.
-
Safety analysis of Oseltamivir and Baloxavir Marboxil after market approval: a pharmacovigilance study based on the FDA adverse event reporting system.BMC Infect Dis. 2024 May 9;24(1):446. doi: 10.1186/s12879-024-09339-4. BMC Infect Dis. 2024. PMID: 38724914 Free PMC article.
-
Post-marketing safety profile of ganirelix in women: a 20-year pharmacovigilance analysis of global adverse drug event databases (2004-2024).BMC Pharmacol Toxicol. 2025 Apr 22;26(1):91. doi: 10.1186/s40360-025-00920-4. BMC Pharmacol Toxicol. 2025. PMID: 40264185 Free PMC article.
-
Adverse drug reactions related to methotrexate: a real-world pharmacovigilance study using the FAERS database from 2004 to 2024.Front Immunol. 2025 Jun 4;16:1586361. doi: 10.3389/fimmu.2025.1586361. eCollection 2025. Front Immunol. 2025. PMID: 40534848 Free PMC article. Review.
-
Clinical efficacy and Safety of Baloxavir Marboxil compared with Oseltamivir against influenza virus in children: A systematic review and meta-analysis.PLoS One. 2025 Jun 23;20(6):e0326777. doi: 10.1371/journal.pone.0326777. eCollection 2025. PLoS One. 2025. PMID: 40549724 Free PMC article.
References
-
- Putri W, Muscatello DJ, Stockwell MS, Newall AT. Economic burden of seasonal influenza in the united States. Vaccine. 2018;36(27):3960–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials