Lack of junctional adhesion molecule (JAM)-B traps CD8 T cells in CNS border zones and ameliorates autoimmune neuroinflammation
- PMID: 40420242
- PMCID: PMC12105289
- DOI: 10.1186/s40478-025-02021-z
Lack of junctional adhesion molecule (JAM)-B traps CD8 T cells in CNS border zones and ameliorates autoimmune neuroinflammation
Abstract
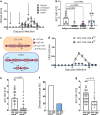
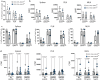
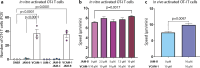
The endothelial blood-brain barrier (BBB) tightly controls T cell entry into the central nervous system (CNS). T cell extravasation across the BBB involves a multi-step cascade with a predominant role of α4β1-integrins. In contrast to CD4 T cells, α4β1-integrin mediated CD8 T cell interaction with the BBB was proposed to involve the tight junction protein junctional adhesion molecule (JAM)-B. Here, we made use of ODC-OVA mice expressing ovalbumin as neo-self-antigen in oligodendrocytes that is solely visible to CD8 T cells, allowing to investigate CD8 T cell-mediated autoimmune neuroinflammation. We generated JAM-B-deficient ODC-OVA mice (ODC-OVA; JAM-BKO mice) and compared CD8 T cell mediated autoimmune neuroinflammation to their ODC-OVA; JAM-BWT littermates. ODC-OVA; JAM-BKO mice developed ameliorated clinical disease, which was associated with a marked reduction in CD8 T cell infiltration into the CNS parenchyma. Surprisingly, lack of JAM-B did not affect CD8 T cell arrest or extravasation in spinal cord microvessels but rather resulted in CD8 T cell accumulation in the subarachnoid space and perivascular spaces in ODC-OVA; JAM-BKO mice. Detection of Jam-2 RNA expression in cells other than BBB endothelial cells contributing to CNS barriers including astrocytes forming the glia limitans, Bergmann glial cells, meningeal fibroblasts and choroid plexus epithelial cells suggests that JAM-B may regulate CD8 T cell entry into the CNS at barriers other than the BBB, particularly at the glia limitans. Thus, targeting JAM-B could provide a therapeutic strategy for treating neuroinflammation without disrupting T cell-mediated immune surveillance in CNS border compartments.
Keywords: BBB; CD8 T cell; CNS; Glia limitans; JAM-B; Neuroinflammation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: All animal experiments were conducted in strict accordance with the regulations and guidelines set by the Veterinary Office of the Canton of Bern, Switzerland. The study was approved by the Veterinary Office of the Canton Bern (permit no. BE31/17, BE55/20, BE73/2021 and BE98/2020), ensuring compliance with ethical standards for animal research. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Abadier M, Haghayegh Jahromi N, Cardoso Alves L, Boscacci R, Vestweber D, Barnum S, Deutsch U, Engelhardt B, Lyck R (2015) Cell surface levels of endothelial ICAM-1 influence the transcellular or paracellular T-cell diapedesis across the blood-brain barrier. European Journal of Immunology, City, pp 1043–1058 - PubMed
-
- Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, Sorokin LM (2006) Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med 203:1007–1019. 10.1084/jem.20051342 - PMC - PubMed
-
- Amatruda M, Chapouly C, Woo V, Safavi F, Zhang J, Dai D, Therattil A, Moon C, Villavicencio J Gordon A, Parkos C, Horng, S(2022) Astrocytic junctional adhesion molecule-A regulates T-cell entry past the glia limitans to promote central nervous system autoimmune attack. Brain Communications, Feb 18;4(2):fcac044. doi: 10.1093/braincomms/fcac044. eCollection 2022. - PMC - PubMed
-
- Arcangeli ML, Frontera V, Bardin F, Obrados E, Adams S, Chabannon C, Schiff C, Mancini SJC, Adams RH, Aurrand-Lions M (2011) JAM-B regulates maintenance of hematopoietic stem cells in the bone marrow. Blood. © 2011 by The American Society of Hematology, City, pp 4609–4619 - PubMed
-
- Arrate MP, Rodriguez JM, Tran TM, Brock TA, Cunningham SA (2001) Cloning of Human Junctional Adhesion Molecule 3 (JAM3) and Its Identification as the JAM2 Counter-receptor. Journal of Biological Chemistry. © 2001 ASBMB. Currently published by Elsevier Inc; originally published by American Society for Biochemistry and Molecular Biology., City, pp 45826–45832 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

