Digital mindfulness-based intervention for people with COPD - a multicentre pilot and feasibility RCT
- PMID: 40420253
- PMCID: PMC12105243
- DOI: 10.1186/s12931-025-03243-4
Digital mindfulness-based intervention for people with COPD - a multicentre pilot and feasibility RCT
Abstract
Background: Mindfulness-based interventions (MBIs) are effective in improving mental and physical health in various chronic conditions. While the GOLD 2024 report recommends MBIs for chronic obstructive pulmonary disease (COPD), scientific evidence in this specific population is scarce. This prospective randomised controlled pilot study investigated the feasibility of an 8-week digital MBI and its preliminary effects on mental and physical health in COPD.
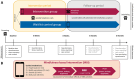
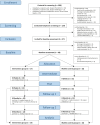
Methods: Psychologically burdened COPD patients (63 ± 7 years, 61% female, FEV1% 41 ± 19) were randomly allocated to the MBI group (n = 14; daily 10-15-minute audio-guided meditation via smartphone) or a waitlist control group (n = 16). Primary outcomes included the intervention's feasibility (dropouts, MBI usage rates, interview and questionnaire responses) and its preliminary effects on symptoms of anxiety and depression (Hospital Anxiety and Depression Scale, HADS). Secondary outcomes included its preliminary effects on the COPD Assessment Test (CAT), Chronic Respiratory Disease Questionnaire (CRQ-SAS), Perceived Stress Scale (PSS-10), and biological stress markers. Exploratory outcomes included momentary subjective stress, anxiety, and dyspnoea after meditating.
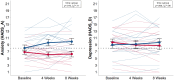
Results: The results indicated that the intervention was feasible (81% usage rate; 93% and 71% found the MBI enjoyable and helpful, respectively), with 21% dropout. A statistically significant intervention (time x group) effect was found for anxiety (HADS-A, p =.010, ηp2 = 0.11) and emotional functioning (CRQ-SAS, p =.004, ηp2 = 0.14), but not for depression (HADS-D, p =.060, ηp2 = 0.06) or any other secondary outcome after 8 weeks. Momentary subjective stress (p <.001, ηp2 = 0.75), anxiety (p =.022, ηp2 = 0.75), and dyspnoea (p <.001, ηp2 = 0.70) were significantly reduced after meditating.
Conclusions: The digital MBI was feasible, with preliminary effects indicating improvements in anxiety and emotional functioning after 8 weeks as well as momentary outcomes after meditating. Future large-scale trials should further assess the effectiveness of digital MBIs in this context. However, the findings suggest that digital MBIs might be promising and effective low-threshold add-on treatments in clinical settings.
Trial registration: The article has been preregistered at ClinicalTrials.gov (identifier: NCT04769505, date: 23rd February 2021).
Keywords: Anxiety; COPD; Depression; Digital mindfulness intervention; Feasibility.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was in accordance with the Declaration of Helsinki, approved by the city of Vienna ethics review board (serial number: EK 20–177 VK), and preregistered at ClinicalTrials.gov (identifier: NCT04769505). All participants provided written informed consent at baseline. Consent for publication: All participants provided consent for publication of their de-identified data. Competing interests: HT was employed as project assistant by the KLI and received lecture fees by Chiesi. GCF and AV are co-heads of the KLI. GCF received lecture fees by AstraZeneca, Boehringer Ingelheim, Chiesi, and Menarini Pharma. FVT received lecture fees by AstraZeneca, Chiesi, and Menarini Pharma.
Figures

References
-
- Matte DL, Pizzichini MMM, Hoepers ATC, Diaz AP, Karloh M, Dias M, Pizzichini E. Prevalence of depression in COPD: A systematic review and meta-analysis of controlled studies. Respir Med. 2016;117:154–61. - PubMed
-
- Chauvet-Gelinier JC, Bonin B. Stress, anxiety and depression in heart disease patients: A major challenge for cardiac rehabilitation. Ann Phys Rehabil Med. 2017;60(1):6–12. - PubMed
-
- Zorn JV, Schür RR, Boks MP, Kahn RS, Joëls M, Vinkers CH. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology. 2017;77:25–36. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

