Spontaneous diuresis in combination with furosemide stress test (SD-FST) as predictor for successful liberation from kidney replacement therapy: a prospective observational study
- PMID: 40420285
- PMCID: PMC12107999
- DOI: 10.1186/s13054-025-05452-1
Spontaneous diuresis in combination with furosemide stress test (SD-FST) as predictor for successful liberation from kidney replacement therapy: a prospective observational study
Abstract
Background: The optimal time for initiating kidney replacement therapy (KRT) in acute kidney injury (AKI) has been extensively studied in recent years. In contrast, there are currently insufficient data on the best time to discontinue KRT. One diagnostic option to unmask tubular reserve and indirectly estimate the glomerular filtration rate is the furosemide stress test (FST).
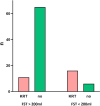
Methods: We conducted a prospective, observational single-center trial. A FST was carried out in patients who developed spontaneous diuresis (SD) during ongoing KRT with a urine output of at least 400 ml in 24 h without any diuretic therapy. A positive FST was defined with urine output > 200 ml within 2 h following intravenous furosemide application. Follow-up was performed for 7 days and the need to restart KRT was assessed daily.
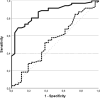
Results: After 100 patients were enrolled in the trial, 98 patients were eligible for further evaluation. 76 patients were FST-positive, while 22 patients were FST-negative. Resumption of KRT within the 7-day follow-up was required in only 14.5% of the FST-positive, but 72.7% of the FST-negative patients (p < 0.001). The urine output after FST was also significantly associated with successful release from KRT (AUC 0.87; p < 0.001).
Conclusions: In critically ill patients with recovery of SD > 400ml/d during ongoing KRT, the FST helps to identify patients who can be successfully liberated from KRT. By detecting the tubular reserve using FST, the possibility of short-term kidney recovery after AKI can be estimated.
Trial registration: German Clinical Trials Registry (DRKS00030560); date of registration 18/11/2022. https://drks.de/search/de/trial/DRKS00030560 .
Keywords: Acute kidney injury (AKI); Furosemide stress test (FST); Kidney replacement therapy (KRT); Recovery of kidney function.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the local Institutional Review Board at the Medical Faculty of the University of Leipzig (171/22-ek). Informed consent to participate was obtained from all of the participants in the study. Patients under 18 years of age were excluded. All experiments were performed in accordance with relevant guidelines and regulations. Consent for publication: Not applicable. Competing interests: Lorenz Weidhase was involved in scientific projects funded by Fresenius Medical Care Deutschland GmbH (Else-Kröner-Straße 1, D-61352 Bad Homburg, Germany) and CytoSorbents Europe GmbH (Müggelseedamm 131, Berlin, Germany). Christina Scharf got speaker honoraria by CytoSorbents Europe GmbH. The remaining authors have disclosed that they do not have any conflicts of interest.
Figures

References
-
- Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–23. - PubMed
-
- Ostermann M, Lumlertgul N, Jeong R, See E, Joannidis M, James M. Acute kidney injury. Lancet. 2025;405(10474):241–56. - PubMed
-
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8. - PubMed
-
- STARRT-AKI Investigators, Canadian Critical Care Trials Group, Australian and New Zealand Intensive Care Society Clinical Trials Group, United Kingdom Critical Care Research Group, Canadian Nephrology Trials Network, Irish Critical Care Trials Group, et al. Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N Engl J Med. 2020;383(3):240–51. 10.1056/NEJMoa2000741 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

