Experimental modeling of Alzheimer's disease: Translational lessons from cross-taxon analyses
- PMID: 40420360
- PMCID: PMC12106051
- DOI: 10.1002/alz.70273
Experimental modeling of Alzheimer's disease: Translational lessons from cross-taxon analyses
Abstract
Alzheimer's disease (AD) is a severely debilitating neurodegenerative disease with a rapidly increasing global prevalence, poorly understood causes, and no efficient treatments. Experimental models are valuable for studying AD pathogenesis, including amyloid beta and tau accumulation, synaptic dysfunction, and neuroinflammation. While no model fully reproduces the disease, we take an evolutionary biology approach to discuss available models across taxa, from mammals (rodents, primates) to zebrafish, Drosophila melanogaster, and Caenorhabditis elegans. Evaluating their strengths and limitations provides insight into disease mechanisms and may refine research strategies for improved diagnostics and therapeutic screening. Traditional models have significantly contributed to AD research, yet their translational limitations highlight the need for physiologically relevant alternatives. Integrating humanized rodent models, zebrafish, organoids, and induced pluripotent stem cell-based systems-along with advances in bioengineering and genetic editing-may offer a more comprehensive framework to bridge the gap between preclinical research and clinical application. HIGHLIGHTS: Experimental models across rodents, primates, zebrafish, fruit flies, and worms provide key insights into Alzheimer's disease (AD). Cross-taxon comparisons assess strengths and weaknesses in AD models. Evolutionary biology approaches refine experimental strategies for AD research. Diverse animal models improve understanding of AD pathogenesis. Cross-species models enhance diagnostics and therapeutic strategy development.
Keywords: Alzheimer's disease; animal models; cross‐taxon analyses; evolutionary psychiatry; translation medicine.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures

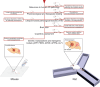

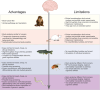
References
-
- Organization WH . Dementia. Accessed 26 December, 2022. https://www.who.int/news‐room/fact‐sheets/detail/dementia#:~:text=Curren...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

