Glycoprotein L-deleted single-cycle rhesus cytomegalovirus vectors elicit MHC-E-restricted CD8+ T cells that protect against SIV
- PMID: 40420384
- PMCID: PMC12396747
- DOI: 10.1093/jimmun/vkaf104
Glycoprotein L-deleted single-cycle rhesus cytomegalovirus vectors elicit MHC-E-restricted CD8+ T cells that protect against SIV
Abstract
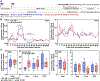
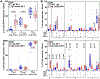
Strain 68-1 rhesus CMV (RhCMV) vectors induce immune responses that mediate early, complete replication arrest of SIV infection in ∼60% of vaccinated rhesus macaques (RMs). This unique efficacy depends on the ability of these vectors to elicit effector memory (EM)-biased CD8+ T cells recognizing SIV peptides presented by MHC-E, rather than MHC-Ia. These efficacious responses still occurred when spread of the 68-1 vector was impaired by deletion of the viral anti-host intrinsic immunity factor phosphoprotein 71 (pp71), but efficacy was lost with a more stringent attenuation strategy based on destabilization of Rh108, the ortholog of the essential human CMV (HCMV) transcription factor UL79 that is required for late viral gene expression. Although unable to produce infectious progeny (ie single-cycle infection), Rh108-deficient vectors elicited durable, high frequency, EM-biased, SIV-specific CD8+ T-cell responses in RMs, but these responses were MHC-Ia-restricted and therefore non-efficacious. Here, we tested a different single-cycle attenuation strategy based on deletion (Δ) of the glycoprotein L (gL) that is essential for viral entry but allows for late gene expression and viral assembly. ΔgL 68-1 RhCMV/SIV vectors, grown on gL-complementing fibroblasts, were robustly immunogenic at doses above 105 PFU, generating high frequency, EM-biased, SIV-specific CD8+ T-cell responses that were also unconventionally restricted, including the MHC-E restriction associated with efficacy. Indeed, these single-cycle vectors manifested replication arrest efficacy in 70% of vaccinated RMs, further linking MHC-E restriction with efficacy, and demonstrating that 68-1 RhCMV/SIV efficacy does not require vector dissemination within the host.
Keywords: CD8+ T cells; CMV vectors; HIV vaccine; MHC-E.
© The Author(s) 2025. Published by Oxford University Press on behalf of The American Association of Immunologists.
Conflict of interest statement
Conflicts of interest
LJP, SGH, DM, and KF are inventors of CMV vector technology licensed to Vir Biotechology. These potential conflicts of interest have been reviewed and managed by OHSU.
Figures



Similar articles
-
Late gene expression-deficient cytomegalovirus vectors elicit conventional T cells that do not protect against SIV.JCI Insight. 2023 Mar 22;8(6):e164692. doi: 10.1172/jci.insight.164692. JCI Insight. 2023. PMID: 36749635 Free PMC article.
-
Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine.Nature. 2011 May 26;473(7348):523-7. doi: 10.1038/nature10003. Epub 2011 May 11. Nature. 2011. PMID: 21562493 Free PMC article.
-
Cytomegaloviral determinants of CD8+ T cell programming and RhCMV/SIV vaccine efficacy.Sci Immunol. 2021 Mar 25;6(57):eabg5413. doi: 10.1126/sciimmunol.abg5413. Sci Immunol. 2021. PMID: 33766849 Free PMC article.
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
-
Programming cytomegalovirus as an HIV vaccine.Trends Immunol. 2023 Apr;44(4):287-304. doi: 10.1016/j.it.2023.02.001. Epub 2023 Mar 7. Trends Immunol. 2023. PMID: 36894436 Free PMC article. Review.
References
-
- Jackson SE, Mason GM, Wills MR. Human cytomegalovirus immunity and immune evasion. Virus Res. 2011;157:151–160. - PubMed
-
- Jarvis MA, Hansen SG, Nelson JA, Picker LJ, Früh K. Vaccine vectors using the unique biology and immunology of cytomegalovirus. In: Reddehase MJ, editor. Cytomegaloviruses: from molecular pathogenesis to intervention. 2nd ed. Caister Academic Press; 2013. p. 469–481.
-
- Kern F et al. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur J Immunol. 1999;29:2908–2915. - PubMed
-
- Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol. 2016;16:367–377. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

