Outcomes in Patients With Resectable Stage III NSCLC Who Did Not Have Definitive Surgery After Neoadjuvant Treatment-A Retrospective Analysis of the SAKK Trials 16/96, 16/00, 16/01, 16/08, and 16/14: A Brief Report
- PMID: 40420867
- PMCID: PMC12104641
- DOI: 10.1016/j.jtocrr.2025.100834
Outcomes in Patients With Resectable Stage III NSCLC Who Did Not Have Definitive Surgery After Neoadjuvant Treatment-A Retrospective Analysis of the SAKK Trials 16/96, 16/00, 16/01, 16/08, and 16/14: A Brief Report
Abstract
Introduction: Neoadjuvant or perioperative treatment, including an immune checkpoint inhibitor (ICI), has emerged as a new standard for patients with resectable stage III NSCLC. Nevertheless, approximately 20% of patients who start neoadjuvant chemo-immunotherapy will not undergo definitive surgery. Little is known about these patients.
Methods: We analyzed outcomes of patients without definitive surgery from five Swiss Group for Clinical Cancer Research (SAKK) trials that investigated different neoadjuvant treatment modalities in patients with resectable stage III-N2 NSCLC. Study treatment included neoadjuvant cisplatin-docetaxel chemotherapy (with or without radiotherapy), either combined with peri-operative durvalumab in the SAKK 16/14 trial (n = 68) or without an ICI (non-ICI trials, n = 431).
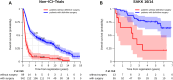
Results: Of the 499 patients, 102 (20%) did not have definitive surgery. Cancellation of surgery occurred in a similar proportion of patients with or without neoadjuvant ICI (19% versus 21%, p = 0.9). Reasons were in non-ICI trials and SAKK 16/14: disease progression (47% and 54%), nonresectability (18% and 8%), medical reasons (17% and 31%), and unknown (18% and 8%), respectively. Of these patients, no patient in SAKK 16/14 and 17 patients (19%) in the non-ICI trials received curative-intended salvage therapy. Three-year overall survival was higher in patients who had definitive surgery compared with those who did not: 78% versus 32% (SAKK 16/14) and 54% versus 10% (non-ICI trials).
Conclusions: In our pooled analysis, patients with definitive surgery had higher survival rates than those without definitive surgery. Prognosis in patients without definitive surgery seems to have improved in the era of ICI.
Keywords: Definitive surgery; Neoadjuvant treatment; Progression; Resectable NSCLC; Salvage treatment.
© 2025 The Authors.
Conflict of interest statement
Dr. Raimann received travel support from 10.13039/100015756Janssen-Cilag, Sanofi, and BeiGene. Dr. Finazzi received honoraria for lectures, presentations, or speakers’ bureaus from AstraZeneca; travel support from 10.13039/100004325AstraZeneca, Astellas, and Debiopharm. Dr. Opitz received honoraria for lectures, presentations, or speakers’ bureaus from AstraZeneca and Roche; and holds positions on advisory boards from AstraZeneca, Merck Sharp & Dohme, Medtronic, and Bristol-Myers Squibb; other financial or nonfinancial interests include Roche (Intuitive - Proctorship) and Medtronic. Dr. Mark received consulting fees from Janssen, Roche, Takeda, Bristol-Myers Squibb, Merck Sharp & Dohme, AstraZeneca, and Merck; and travel support from Janssen, AstraZeneca, Roche, Takeda, and Amgen. Dr. Addeo received grants from AstraZeneca; honoraria for lectures, presentations, or speakers’ bureaus from AstraZeneca, Eli Lilly, and Amgen; and holds positions on advisory boards from Bristol-Myers Squibb, AstraZeneca, Roche, Astellas, Novartis, Merck Sharp & Dohme, Pfizer, Eli Lilly, Amgen, and Merck. Dr. Mauti received grants from AstraZeneca and Gilead; honoraria for lectures, presentations or speakers’ bureaus from Amgen; travel support from AstraZeneca, Roche and Sanofi; and holds positions on advisory boards from Takeda, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck, Sanofi, Novartis, AstraZeneca, Pfizer, Regeneron, Daiichi Sankyo and Sanofi. Dr. Früh received consulting fees (all to institution) from Bristol-Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Boehringer Ingelheim, Roche, Takeda, Pfizer, Janssen, Daiichi Sankyo, and PharmaMar. Dr. Rothschild holds research grants from AstraZeneca, Merck, Serono, Roche and Amgen; received honoraria for lectures, presentations or speakers bureaus from Roche, Bristol-Myers Squibb, AstraZeneca, Amgen, Merck Sharp & Dohme, Novartis, Roche Diagnostics and Takeda; honoraria for expert testimony from Roche, AstraZeneca and Bristol-Myers Squibb; travel support from 10.13039/100004337Roche, Eli Lilly, 10.13039/100002491Bristol-Myers Squibb, Amgen, AstraZeneca and Merck Sharp & Dohme; and holds positions on advisory boards from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Merck Sharp & Dohme, Novartis, Otsuka, Pfizer, PharmaMar, Roche Pharma, Bristol-Myers Squibb and Takeda; other nonfinancial interests include Member/Vice-President from the Swiss Group for Clinical Cancer Research (SAKK) and Member (elected Member) from the Swiss Federal Drug Commission (Federal Health Office). Dr. Pless received consulting fees from AbbVie, AstraZeneca, Bristol-Myers Squibb, Roche, Takeda, Eisai Pharma, Merck Sharp & Dohme, Novartis, Pfizer and Merck; honoraria for lectures, presentations or speakers’ bureaus from Janssen, Bayer, Nestlé, Sanofi and Amgen; travel support from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Roche, Takeda and Vifor. Dr. König received institutional grants from Geistlich-Stucki Stiftung; consulting fees from AstraZeneca, Merck Sharp & Dohme und Novartis; honoraria for lectures, presentations or speakers’ bureaus from Amgen, SanofiSanofi, Swiss Oncology in Motion and Mirati; travel support from 10.13039/100004339Sanofi, 10.13039/100002429Amgen and Roche; and holds positions on advisory boards from AstraZeneca, PharmaMar, Merck Bristol-Myers Squibb and Merck Sharp & Dohme. The remaining authors declare no conflict of interest.
Figures

References
-
- Heymach J.V., Harpole D., Mitsudomi T., et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389:1672–1684. - PubMed
-
- Cascone T., Awad M.M., Spicer J.D., et al. Perioperative nivolumab in resectable lung cancer. N Engl J Med. 2024;390:1756–1769. - PubMed
-
- Liu J., Blake S.J., Yong M.C., et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6:1382–1399. - PubMed
LinkOut - more resources
Full Text Sources

