Recent Advances in HPV Detection: From Traditional Methods to Nanotechnology and the Application of Quantum Dots
- PMID: 40420910
- PMCID: PMC12104828
- DOI: 10.2147/IJN.S524518
Recent Advances in HPV Detection: From Traditional Methods to Nanotechnology and the Application of Quantum Dots
Abstract
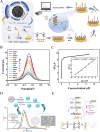
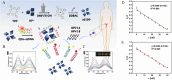
Cervical cancer, a significant public health concern, demands precise and expeditious detection methods to curb the spread of human papillomavirus (HPV). The early detection of cervical cancer remains a critical challenge in developing reliable and efficient screening tools to meet the demand for controlling cervical cancer. Traditional detection techniques are often cumbersome, costly, and inadequate for on-site HPV testing. Nanotechnology, with its unique electrical, chemical, and optical properties, has emerged as a pivotal component in the development of biosensors for rapid and reliable HPV detection. This article provides a comprehensive review of the advancements in cervical cancer detection, encompassing traditional methods, emerging protocols, and novel quantum dots (QDs)-based approaches for detection. The review examines the application of various nanomaterials in electrochemical and photoelectrochemical biosensors for the diagnosis of cervical cancer, with these innovations offering a significant improvement over conventional approach. Furthermore, we detail the synthesis methods of QDs and their properties, illustrate the substantial enhancement in sensor performance achieved through their applications, and elucidate the improvements and challenges associated with these new protocols while highlighting the potential application prospects of novel QDs technology in HPV detection.
Keywords: biosensors; cervical cancer; early diagnosis; human papillomavirus detection; nanotechnology; quantum dots.
© 2025 He et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures


Similar articles
-
Advances in early diagnosis of cervical cancer based on biosensors.Biotechnol Bioeng. 2022 Sep;119(9):2305-2312. doi: 10.1002/bit.28149. Epub 2022 Jun 16. Biotechnol Bioeng. 2022. PMID: 35665484 Review.
-
Electrochemical chip-based genomagnetic assay for detection of high-risk human papillomavirus DNA.Biosens Bioelectron. 2016 Sep 15;83:300-5. doi: 10.1016/j.bios.2016.04.035. Epub 2016 Apr 14. Biosens Bioelectron. 2016. PMID: 27132004
-
Advances in human papillomavirus detection for cervical cancer screening and diagnosis: challenges of conventional methods and opportunities for emergent tools.Anal Methods. 2025 Feb 13;17(7):1428-1450. doi: 10.1039/d4ay01921k. Anal Methods. 2025. PMID: 39775553 Free PMC article. Review.
-
Advancements in electrochemical DNA sensor for detection of human papilloma virus - A review.Anal Biochem. 2018 Sep 1;556:136-144. doi: 10.1016/j.ab.2018.07.002. Epub 2018 Jul 5. Anal Biochem. 2018. PMID: 29981317 Review.
-
Nanostructured genosensor platform based on polypyrrole film and graphene quantum dots for the detection of high-risk HPV.J Pharm Biomed Anal. 2025 Sep 15;263:116920. doi: 10.1016/j.jpba.2025.116920. Epub 2025 Apr 24. J Pharm Biomed Anal. 2025. PMID: 40311543
References
-
- Falcaro M, Castañon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. 2021;398(10316):2084–2092. doi:10.1016/S0140-6736(21)02178-4 - DOI - PubMed

