Preparation, and ex vivo and in vivo Characterization of Favipiravir-Loaded Aspasomes and Niosomes for Nose-to-Brain Administration
- PMID: 40420912
- PMCID: PMC12105672
- DOI: 10.2147/IJN.S518486
Preparation, and ex vivo and in vivo Characterization of Favipiravir-Loaded Aspasomes and Niosomes for Nose-to-Brain Administration
Abstract
Purpose: The present study aimed to develop and compare the intranasal applicability of favipiravir-loaded aspasomes (FAV-ASPs) using film hydration method, and favipiravir-loaded niosomes (FAV-NIOs) using ethanol injection method.
Methods: The FAV-ASP and FAV-NIO formulations were characterized according to nanoparticulate characteristics (DLS, drug loading, drug encapsulation efficacy, droplet size distribution), drug release and permeability behavior.
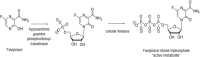
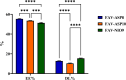
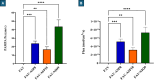
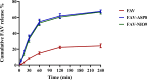
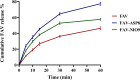
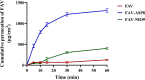
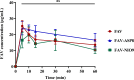
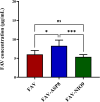
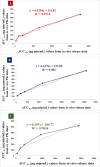
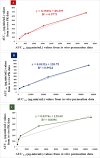
Results: The optimized FAV-ASP formulation (FAV-ASP8) consisted of FAV, ascorbyl palmitate, Span® 60 and cholesterol (30:25:25:50 w/w) with nano-scale size range (292.06 ± 2.10 nm), narrow polydispersity index (PDI) value (0.36 ± 0.03), adequate zeta potential (-74.73 ± 3.28 mV) and acceptable encapsulation efficiency (55.33 ± 0.41%). The optimized FAV-NIO formulation (FAV-NIO9) contained FAV, Span® 60 and cholesterol (30:30:40 w/w) with nano-scale size range (167.13 ± 1.60 nm), narrow PDI value (0.07 ± 0.01), adequate zeta potential (-27.1 ± 1.24 mV) and acceptable encapsulation efficiency (51.30 ± 0.69%). FAV-ASP8 and FAV-NIO9 were suitable for spraying into the nasal cavity (droplet size distribution <200 µm). In vitro drug release and permeability studies demonstrated enhanced solubility and increased blood-brain barrier (BBB) permeability of FAV formulations, respectively. The ex vivo human nasal permeability study revealed that FAV diffusion from FAV-ASP8 was higher than from FAV-NIO9 or initial FAV. Furthermore, the in vivo animal study showed that FAV-ASP8 had a higher BBB penetration compared to FAV-NIO9 and pure FAV. The in vitro-in vivo correlation study showed good correlation between the in vitro and the in vivo pharmacokinetic data.
Conclusion: FAV-ASP8 for nose-to-brain delivery system could be a promising formulation to improve FAV bioavailability compared to FAV-NIO9.
Keywords: aspasomes; ex vivo nasal permeability; favipiravir; in vivo nasal permeability; niosomes; nose-to-brain delivery.
© 2025 Salamah et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Formulation and Evaluation of Niosomal in situ Nasal Gel of a Serotonin Receptor Agonist, Buspirone Hydrochloride for the Brain Delivery via Intranasal Route.Pharm Nanotechnol. 2018;6(1):69-78. doi: 10.2174/2211738506666180130105919. Pharm Nanotechnol. 2018. PMID: 29380709
-
Favipiravir-Based Ionic Liquids as Potent Antiviral Drugs for Oral Delivery: Synthesis, Solubility, and Pharmacokinetic Evaluation.Mol Pharm. 2021 Aug 2;18(8):3108-3115. doi: 10.1021/acs.molpharmaceut.1c00324. Epub 2021 Jul 10. Mol Pharm. 2021. PMID: 34250805
-
A promise of nose to brain delivery of bevacizumab intranasal sol-gel formulation substantiated in rat C6 glioma model.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr;398(4):4123-4148. doi: 10.1007/s00210-024-03536-3. Epub 2024 Oct 17. Naunyn Schmiedebergs Arch Pharmacol. 2025. Retraction in: Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):7653. doi: 10.1007/s00210-025-04177-w. PMID: 39417842 Retracted.
-
Non-Invasive Techniques of Nose to Brain Delivery Using Nanoparticulate Carriers: Hopes and Hurdles.AAPS PharmSciTech. 2024 Oct 30;25(8):256. doi: 10.1208/s12249-024-02946-z. AAPS PharmSciTech. 2024. PMID: 39477829 Review.
-
Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs.J Control Release. 2018 Jul 10;281:139-177. doi: 10.1016/j.jconrel.2018.05.011. Epub 2018 May 24. J Control Release. 2018. PMID: 29772289 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

