PCOS and Inositols - Advances and Lessons We are Learning. A Narrative Review
- PMID: 40420946
- PMCID: PMC12104671
- DOI: 10.2147/DDDT.S524718
PCOS and Inositols - Advances and Lessons We are Learning. A Narrative Review
Abstract
Introduction: This Expert Opinion covers recent updates in the use of Inositol in polycystic ovary syndrome (PCOS), highlighting the specific effects triggered upon ovarian steroidogenesis.
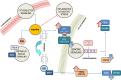
Areas covered: An impressive body of evidence, obtained from molecular, animal and clinical studies, demonstrated the striking association between PCOS and the metabolism of myo-Inositol (myo-Ins) and its isomer D-Chiro-Inositol (DCI). Early investigations focused primarily on the metabolic consequences of inositol in modulating insulin transduction. However, recent advances disclosed that Inositols trigger direct effects on steroidogenesis. High DCI levels exacerbate androgen synthesis, and downregulate aromatase expression. Myo-Ins modulates insulin effects too, but exerts opposite actions on steroidogenesis, by increasing aromatase and FSH receptor expression. Clinical studies demonstrated myo-Ins efficacy, suggesting that an appropriate ratio in between myo-Ins/DCI (40:1) improves the reproductive function in PCOS women, even in absence of insulin resistance.
Expert opinion: Inositol-based treatments in PCOS are gaining momentum, demonstrating safety and efficacy greater than those obtained with other pharmacological agents. The efficacy depends not only on the modulation of insulin sensitivity but also on the direct, steroidogenic effects upon the ovaries. Adequate adsorption of Inositol is a critical issue, and the association of α-Lactalbumin can significantly overcome this problem. However, if a treatment based on inositol could be equally effective on different phenotypes of PCOS needs a specific assessment.
Keywords: (polycystic ovary syndrome); D-chiro-inositol; PCOS; aromatase; hyperandrogenism; insulin resistance; myo-Inositol.
Plain language summary
Myo-inositol (myo-Ins) and D-chiro-inositol (DCI) are two isomers of inositol playing a key role in insulin signaling throughout the body, mainly through their phosphoglycan derivatives. They act as important regulators of hormone production within the ovaries, modulating steroidogenesis.Molecular and clinical studies showed that myo-Ins enhances FSH and aromatase activity, while DCI stimulates androgenesis in the theca. These isomers seem to have opposite, yet complementary, actions upon ovarian function. In the last decades, inositols have emerged to treat Polycystic Ovary Syndrome (PCOS), one of the most common benign ovarian disorders affecting young, fertile women.Myo-Ins/DCI ratio in the blood averages 40:1, and this parameter has been used as a basis for establishing a pharmacological formula. Treatments based according to this ratio showed to be successful in the management of several symptoms and signs of PCOS. This is particularly evident in obese patients, whereby the low doses of DCI help in counteracting insulin resistance.Further extensive research, both at the molecular and clinical levels, is needed to better understand inositol biology in mammals. In particular, it should be clarified how myo-inositol modulates the nuclear steroidogenic factor-1 (SF-1) and other key enzymes involved in steroid production. This could be done through epigenetic modifications.
© 2025 Lentini et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29(2):181–191. doi:10.1016/S0002-9378(15)30642-6 - DOI
-
- Miazgowski T, Martopullo I, Widecka J, Miazgowski B, Brodowska A. National and regional trends in the prevalence of polycystic ovary syndrome since 1990 within Europe: the modeled estimates from the Global Burden of Disease Study 2016. Arch Med Sci. 2021;17(2):343–351. doi:10.5114/aoms.2019.87112 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

