Collagen mimetic peptides as novel therapeutics for vascular disease in the central nervous system
- PMID: 40421131
- PMCID: PMC12104236
- DOI: 10.3389/fnins.2025.1569347
Collagen mimetic peptides as novel therapeutics for vascular disease in the central nervous system
Abstract
Background: Loss of vascular integrity is a common comorbidity of neurodegenerative diseases of the central nervous system (CNS). Compromised blood flow to the brain and excessive vascular remodeling is evident in chronic systemic cardiovascular diseases such as atherosclerosis, driving neurodegeneration and subsequent cognitive decline. Vascular remodeling occurs in response to changes in the microenvironment, with the extracellular matrix (ECM) as a major component. Collagens within the ECM and vascular basement membrane are integral to endothelial cell (EC) function and maintenance of the blood-brain barrier. Disruption of the ECM and breakdown of collagen with disease may lead to vascular dysfunction and neurodegeneration.
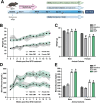
Methods: We induced hyperglycemia in ApoE-deficient (ApoE-/-) mice by intraperitoneal injection of streptozocin (STZ; 50 mg/Kg) for 5 days and accelerated diabetic atherosclerotic disease through a high fat diet (HFD). Over a 12 weeks period, mice received weekly intravenous treatment of collagen mimetic peptide (CMP) or vehicle (phosphate buffered saline) to assess efficacy in promoting vascular integrity in central brain structures.
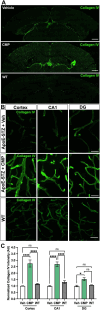
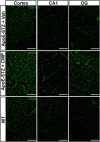
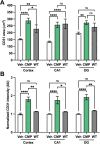
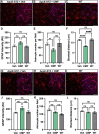
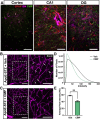
Results: Following the STZ/HFD regimen, diabetic atherosclerotic ApoE-/- mice treated with CMP exhibited increased vascular integrity compared to vehicle in the cortex and in the CA1 and dentate gyrus regions of the hippocampus, as assed by higher levels of the endothelial cell adhesion glycoprotein CD31 and intravascular collagen IV, increased vascular area, and diminished leakage. Interestingly, in hippocampus, astrocytes were closer in proximity to vessels despite being less numerous in the CMP group.
Conclusion: Collagen integrity is important for maintaining cerebrovascular architecture in disease. Application of CMP which intercalates with and repairs damaged collagen may have therapeutic use in neurodegenerative diseases by preserving vasculature structure and promoting blood-brain barrier integrity. These findings underscore the need to further explore the role of collagen repair as a novel therapeutic for diseases of the brain involving vascular degradation.
Keywords: atherosclerosis; cerebrovascular disease; collagen; collagen mimetic peptides; diabetes; extracellular matrix; neurodegeneration; vascular dysfunction.
Copyright © 2025 Bossardet, Holden, Del Buono, Schlumpf, Wareham and Calkins.
Conflict of interest statement
ES and BD were employed by Sailfish Therapeutics, LLC. DC was a consultant for Sailfish Therapeutics, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Airhart N., Brownstein B. H., Cobb J. P., Schierding W., Arif B., Ennis T. L., et al. (2014). Smooth muscle cells from abdominal aortic aneurysms are unique and can independently and synergistically degrade insoluble elastin. J. Vasc. Surg. 60 1033–1041. 10.1016/j.jvs.2013.07.097 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

