Dynamics of stress-induced c-fos expression in the rat prelimbic cortex: lessons from intronic and mature RNA and protein analyses
- PMID: 40421146
- PMCID: PMC12104749
- DOI: 10.1016/j.ynstr.2025.100729
Dynamics of stress-induced c-fos expression in the rat prelimbic cortex: lessons from intronic and mature RNA and protein analyses
Abstract
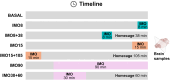
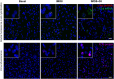
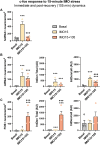
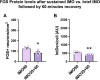
Despite the extensive use of c-fos as a marker of stress-induced neuronal activation, key aspects regarding its dynamics of expression remain poorly characterized. In the present study, we assessed in the prelimbic cortex of adult male rats the immediate transcriptional response of c-fos by measuring the heteronuclear (hn)RNA and mature (m)RNA expression by double fluorescent in situ hybridization as well as the c-Fos protein using immunofluorescence (FOS). We quantified in three different experiments the number of c-fos hnRNA+, mRNA+ and FOS+ neurons under basal conditions, immediately after different periods of immobilization stress (IMO), and after a recovery period. Our results indicate that stress induced a large increase in the number of positive neurons for all markers analyzed, each displaying a different time course. Moreover, our findings indicate that measuring the intensity of signal per neuron also provides relevant information. In addition, we report an increased number of FOS+ neurons after only 8-15 min of IMO, suggesting a surprisingly fast initiation of protein translation. Finally, the maturation from c-fos hnRNA+ to mRNA+ might depend on the duration and/or intensity of stress-induced activation. Our findings contribute to a better understanding of the dynamics of stress-induced c-fos expression and underscore the importance of examining multiple molecular components when using c-fos as a proxy of neuronal activation.
Keywords: Immobilization stress; Neuronal activation; Prelimbic cortex; Stress duration; c-fos expression.
© 2025 The Authors. Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing financial interest or potential conflict of interest.
Figures


Similar articles
-
Long-term effects of a single exposure to immobilization stress on the hypothalamic-pituitary-adrenal axis: transcriptional evidence for a progressive desensitization process.Eur J Neurosci. 2003 Sep;18(6):1353-61. doi: 10.1046/j.1460-9568.2003.02857.x. Eur J Neurosci. 2003. PMID: 14511316
-
Immediate-early gene response to repeated immobilization: Fos protein and arc mRNA levels appear to be less sensitive than c-fos mRNA to adaptation.Eur J Neurosci. 2010 Jun;31(11):2043-52. doi: 10.1111/j.1460-9568.2010.07242.x. Eur J Neurosci. 2010. PMID: 20608965
-
Restraint-induced fra-2 and c-fos expression in the rat forebrain: relationship to stress duration.Neuroscience. 2007 Dec 5;150(2):478-86. doi: 10.1016/j.neuroscience.2007.09.013. Epub 2007 Sep 14. Neuroscience. 2007. PMID: 17936518 Free PMC article.
-
Stress-induced activation of neuronal activity and corticotropin-releasing factor gene expression in the paraventricular nucleus is modulated by glucocorticoids in rats.J Clin Invest. 1995 Jul;96(1):231-8. doi: 10.1172/JCI118026. J Clin Invest. 1995. PMID: 7615792 Free PMC article.
-
C-fos mRNA pattern and corticotropin-releasing factor neuronal activity throughout the brain of rats injected centrally with a prostaglandin of E2 type.J Neuroimmunol. 1996 Nov;70(2):163-79. doi: 10.1016/s0165-5728(96)00114-2. J Neuroimmunol. 1996. PMID: 8898725
References
-
- Armario A. Immediate Early Genes in Sensory Processing, Cognitive Performance and Neurological Disorders. Springer US; 2006. The contribution of immediate early genes to the understanding of brain processing of stressors; pp. 199–221. - DOI
-
- Bisler S., Schleicher A., Gass P., Stehle J.H., Zilles K., Staiger J.F. Expression of c-Fos, ICER, Krox-24 and JunB in the whisker-to-barrel pathway of rats: time course of induction upon whisker stimulation by tactile exploration of an enriched environment. J. Chem. Neuroanat. 2002;23(3):187–198. doi: 10.1016/S0891-0618(01)00155-7. - DOI - PubMed
-
- Bonapersona V., Schuler H., Damsteegt R., Adolfs Y., Pasterkamp R.J., van den Heuvel M.P., Joëls M., Sarabdjitsingh R.A. The mouse brain after foot shock in four dimensions: temporal dynamics at a single-cell resolution. Proc. Natl. Acad. Sci. U. S. A. 2022;119(8) doi: 10.1073/pnas.2114002119. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

