Venous access devices (Review)
- PMID: 40421227
- PMCID: PMC12105099
- DOI: 10.3892/mi.2025.241
Venous access devices (Review)
Abstract
Venous access devices can be categorized based on the termination site of the tip of the catheter into central and peripheral access devices. Selecting the type of venous access device depends on various factors, including the condition of the patient, the anticipated duration of therapy, the use of vesicant or hyperosmolar therapies and the potential risk of complications. Peripheral intravenous catheters provide adequate venous access in the majority of hospitalized patients; however, their use is associated with high failure rates. Non-tunneled central venous access catheters are typically used in critically ill patients and are ideally suited for short-term use, while tunneled central catheters are utilized in those who require long-term intravenous therapy due to their extended dwell times. Each of these devices has unique characteristics requiring an in-depth understanding of the indications and current evidence-based recommendations to optimize their use. The present narrative review aimed to describe the different types of venous access devices and recommendations for their use based on current evidence-based recommendations. A narrative review of the literature was performed based on searches of the PubMed and Google Scholar database from 1990 through November 1, 2024. The type of device used, the insertion site, patient characteristics and clinical needs, and the risk of complications are factors that a provider needs to consider when ordering the placement of a venous access device are discussed. The present review also discusses the prevention of negative adverse events, such as bloodstream infections and thrombosis, associated with specific devices. In addition, current evidence-based recommendations for device selection and indications for use are described. The present narrative review provides a detailed examination of venous access devices that are essential in the care and treatment of patients. Since venous access is associated with inherent risks, device selection and meticulous post-insertion care are paramount in ensuring patient safety and successful therapy.
Keywords: non-tunneled central venous access catheters; peripheral intravenous catheters; tunneled central venous access catheters; venous access devices.
Copyright: © 2025 Abdulelah et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

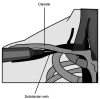
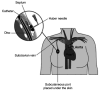
References
-
- Pittirut M, Van Boxtel T, Scoppettuolo G, Carr P, Konstantinou E, Ortiz Miluy G, Lamperti M, Goossens GA, Simcock L, Dupont C, et al. European recommendations on the proper indication and use of peripheral venous access devices (the ERPIUP consensus): A WoCoVA project. J Vasc Access. 2023;24:165–182. doi: 10.1177/11297298211023274. - DOI - PubMed
-
- Moureau NL, Alexandrou E. Device Selection. In: Vessel Health and Preservation: The Right Approach for Vascular Access. Moureau NL (ed). Springer International Publishing: Cham, pp23-41, 2019.
-
- Pattnaik SK, Sahoo S, Hota B. Establishment of peripherally inserted central catheter line clinic at a tertiary care hospital: Review of first 100 Cases. Apollo Med. 2023;20:341–345.
-
- Beecham GB, Tackling G. Peripheral Line Placement. In: StatPearls. StatPearls Publishing, Treasure Island, FL, 2025. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
