Single-Center Evaluation of the Myval Balloon-Expandable Transcatheter Heart Valve: A Follow-Up Study: A Retrospective Cohort Study
- PMID: 40421254
- PMCID: PMC12104823
- DOI: 10.1002/hsr2.70808
Single-Center Evaluation of the Myval Balloon-Expandable Transcatheter Heart Valve: A Follow-Up Study: A Retrospective Cohort Study
Abstract
Background and aims: Our objective is to report our single-center experience with the novel balloon-expandable Myval Transcatheter Heart Valve (THV) system in Transcatheter Aortic Valve Replacement (TAVR) procedures.
Methods: We conducted a retrospective study on a cohort of consecutive patients who underwent TAVR utilizing Myval THV from September 2021 to August 2023 at a tertiary care cardiac center. We collected baseline characteristics, pre- and post-procedural echocardiographic findings, procedural details, in-hospital outcomes, VARC-3 technical success, and complications. Additionally, patients were followed up for 3 months concerning their clinical outcomes.
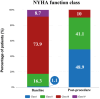
Results: The study population comprised 92 TAVR patients with a mean age of 76.8 ± 7.3 years, 66.3% were male, and the mean STS score was 5.9 ± 3.2%. The most common valve sizes used were 24.5 mm (30.4%), 23 mm (26.1%), and 27.5 mm (17.4%). Pre-dilation was performed in 32 cases (34.8%), achieving a 93.5% technical success rate. In-hospital mortality occurred in three patients (3.3%), which included one annulus rupture. Permanent pacemaker implantation was required in six patients (6.5%). Three patients (3.3%) exhibited 3+ paravalvular leakage demonstrated by angiography. The New York Heart Association (NYHA) functional class showed significant improvement from baseline to discharge (p < 0.0001). At the 3-month follow-up, five patients encountered mortality (5.4), and three experienced an episode of stroke or transient ischemic attack (3.2%). Two other patients were hospitalized due to cardiovascular events during the 3-month follow-up.
Conclusion: The Myval THV shows a favorable safety and efficacy profile in TAVR, with low mortality and complications at 3 months.
Keywords: Myval; aortic stenosis; balloon‐expandable; transcatheter aortic valve replacement; transcatheter heart valve.
© 2025 The Author(s). Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
S. Abdi has a role as a clinical proctor for the Sapien 3 valve by Edwards Lifesciences. E. Khalilipur is certified as an independent operator in transcatheter heart valve replacements with the Myval THV system. A. Firouzi is an international proctor for Myval THV. The remaining authors declare no conflicts of interest. Supporting sources were not involved in study design, data collection, analysis, interpretation, writing the report, or deciding to submit the report for publication.
Figures

Similar articles
-
Clinical Comparison of a Novel Balloon-Expandable Versus a Self-Expanding Transcatheter Heart Valve for the Treatment of Patients with Severe Aortic Valve Stenosis: The EVAL Registry.J Clin Med. 2022 Feb 12;11(4):959. doi: 10.3390/jcm11040959. J Clin Med. 2022. PMID: 35207232 Free PMC article.
-
The Myval Balloon-Expandable Transcatheter Heart Valve Implant in Aortic and Mitral Interventions: A Single-Center Experience.Cureus. 2025 Mar 15;17(3):e80638. doi: 10.7759/cureus.80638. eCollection 2025 Mar. Cureus. 2025. PMID: 40236357 Free PMC article.
-
Single-Center Experience with the Balloon-Expandable Myval Transcatheter Aortic Valve System in Patients with Bicuspid Anatomy: Procedural and 30-Day Follow-Up.J Clin Med. 2024 Jan 17;13(2):513. doi: 10.3390/jcm13020513. J Clin Med. 2024. PMID: 38256647 Free PMC article.
-
A novel balloon-expandable transcatheter aortic valve bioprosthesis: Myval and Myval Octacor.Expert Rev Cardiovasc Ther. 2024 Jul;22(7):325-337. doi: 10.1080/14779072.2024.2375345. Epub 2024 Jul 15. Expert Rev Cardiovasc Ther. 2024. PMID: 38970466 Review.
-
Myval: A Novel Transcatheter Heart Valve for the Treatment of Severe Aortic Stenosis.Interv Cardiol. 2023 Apr 10;18:e12. doi: 10.15420/icr.2020.32. eCollection 2023. Interv Cardiol. 2023. PMID: 37398875 Free PMC article. Review.
References
-
- Prendergast B. D., Baumgartner H., Delgado V., et al., “Transcatheter Heart Valve Interventions: Where Are We? Where Are We Going?,” European Heart Journal 40, no. 5 (2019): 422–440. - PubMed
-
- Holzamer A., Bedogni F., van Wyk P., et al., “Performance of the 32 mm Myval Transcatheter Heart Valve for Treatment of Aortic Stenosis in Patients With Extremely Large Aortic Annuli in Real‐World Scenario: First Global, Multicenter Experience,” Catheterization and Cardiovascular Interventions 102, no. 7 (2023): 1364–1375. - PubMed
-
- Mack M. J., Leon M. B., Thourani V. H., et al., “Transcatheter Aortic‐Valve Replacement With a Balloon‐Expandable Valve in Low‐Risk Patients,” New England Journal of Medicine 380, no. 18 (2019): 1695–1705. - PubMed
-
- Popma J. J., Deeb G. M., Yakubov S. J., et al., “Transcatheter Aortic‐Valve Replacement With a Self‐Expanding Valve in Low‐Risk Patients,” New England Journal of Medicine 380, no. 18 (2019): 1706–1715. - PubMed
LinkOut - more resources
Full Text Sources

