A Thorough Characterization of the Tellurocyanate Anion
- PMID: 40421504
- PMCID: PMC12304862
- DOI: 10.1002/anie.202507543
A Thorough Characterization of the Tellurocyanate Anion
Abstract
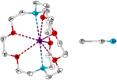
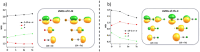
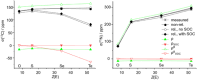
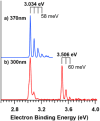
Tellurocyanate, [TeCN]-, is the heaviest group 16 congener of the cyanate anion, [OCN]-. Due to the relative instability of the C─Te bond, tellurocyanate chemistry has seen only scarce attention. Here, we present the facile synthesis and thorough characterization of [K@crypt-222][TeCN]. The anion is essentially linear with interatomic distances C─N = 1.150(6)Å and C─Te = 2.051(4)Å, thus approximating a C≡N triple bond and for C─Te a bond order between 1 and 2. Fully 13C and 15N labeled [Te13C15N]- allowed for the extraction of chemical shifts and all possible coupling constants (13C = 77.8 ppm, 15N = 285.7 ppm, 125Te = -566 ppm, 1J13C-15N = 8 Hz, 1J13C-125Te = 748 Hz, 2J15N-125Te = 55 Hz), which were also determined independently by quantum chemical calculations. In the series [ChCN]- (Ch = O─Te), [TeCN]- shows the strongest spin-orbit coupling (SOC) induced heavy-atom effect on the light-atom shielding (SO-HALA-effect). In contrast, 15N shifts are also well described without considering relativistic effects and/or SOC. Negative-ion photoelectron spectroscopy was used to extract the electron affinity (EA = 3.034 eV) and spin-orbit splitting (3807 cm-1) of [TeCN]•. These values continue the trends of falling EA and rising SOC in the series [ChCN]•.
Keywords: Bonding analysis; Cyanates; DFT; Negative ion photoelectron spectroscopy; Nuclear magnetic resonance.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
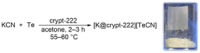
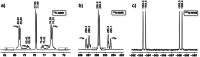
Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
Cited by
-
Historical and Recent Developments in the Chemistry of Cyanate Congeners.Inorg Chem. 2025 Jul 7;64(26):12900-12917. doi: 10.1021/acs.inorgchem.5c01041. Epub 2025 Jun 11. Inorg Chem. 2025. PMID: 40498324 Free PMC article. Review.
References
-
- Birckenbach L., Kellermann K., Ber. dtsch. Chem. Ges. A/B 1925, 58, 786–794.
-
- Becker G., Schwarz W., Seidler N., Westerhausen M., Z. Anorg. Allg. Chem. 1992, 612, 72–82.
-
- Becker G., Hübler K., Z. Anorg. Allg. Chem. 1994, 620, 405–417.
-
- Tambornino F., Hinz A., Köppe R., Goicoechea J. M., Angew. Chem., Int. Ed. 2018, 57, 8230–8234. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

