Oxygen supply is a prerequisite for response to aluminum in cultured cells of tobacco (Nicotiana tabacum)
- PMID: 40421753
- PMCID: PMC12344093
- DOI: 10.1093/pcp/pcaf055
Oxygen supply is a prerequisite for response to aluminum in cultured cells of tobacco (Nicotiana tabacum)
Abstract
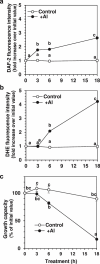
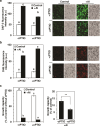
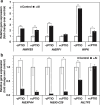
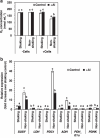
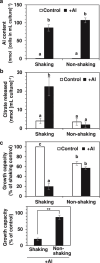
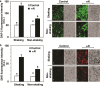
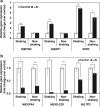
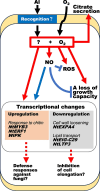
Responses to aluminum (Al) were investigated in tobacco cells (cell line SL) in a calcium-sucrose solution for up to 24 h under shaking (aerobic) condition. Microarray analysis of upregulated and downregulated genes under Al exposure and following Gene Ontology (GO) enrichment analysis of biological process category revealed only one GO term to be enriched for the upregulated genes, "response to chitin," annotated with genes encoding transcription factors (NtERF1 and NtMYB3) and MAP kinase (WIPK), and nine GO terms for the downregulated genes, including "cell wall loosening" and "lipid transport," annotated with genes encoding expansin (NtEXPA4) and lipid transfer protein (LTP)/LTP-like (NtLTP3 and NtEIG-C29), respectively. Al triggered the production of nitric oxide (NO) then reactive oxygen species (ROS). Addition of NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide decreased the levels of NO and a part of the transcriptional changes described above, but increased the levels of ROS and a loss of growth capacity, suggesting a role of the NO to induce the transcriptional changes partly and to repress these toxic responses under Al exposure. Under non-shaking (anaerobic) condition, the cells exhibited upregulation of several hypoxia-responsive genes. The cells exposed to Al exhibited the same level of Al accumulation but much lower levels of the Al responses including NO production, ROS production, a loss of growth capacity, citrate secretion, and a part of the transcriptional changes described above, compared with the cells under shaking condition. These results suggest that coexistence of oxygen with Al is necessary to trigger the Al responses related to toxicity and tolerance.
Keywords: aluminum toxicity; aluminum-responsive genes; cell wall loosening; chitin-responsive genes; dioxygen; hypoxia.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Japanese Society of Plant Physiologists.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts.Crit Rev Toxicol. 2014 Oct;44 Suppl 4(Suppl 4):1-80. doi: 10.3109/10408444.2014.934439. Crit Rev Toxicol. 2014. PMID: 25233067 Free PMC article.
-
Comparative Transcriptome Reveals ART1-Dependent Regulatory Pathways for Fe Toxicity Response in Rice Roots.Physiol Plant. 2025 Jul-Aug;177(4):e70398. doi: 10.1111/ppl.70398. Physiol Plant. 2025. PMID: 40653961 Free PMC article.
-
Therapeutic effects of PDGF-AB/BB against cellular senescence in human intervertebral disc.Elife. 2025 Jul 16;13:RP103073. doi: 10.7554/eLife.103073. Elife. 2025. PMID: 40668091 Free PMC article.
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article.
References
-
- Akaike, T., Yoshida, M., Miyamoto, Y., Sato, K., Kohno, M., Sasamoto, K., et al. (1993) Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry 32:827–832. - PubMed
-
- Baier, R., Schiene, K., Kohring, B., Flaschel, E., Niehaus, K. (1999) Alfalfa and tobacco cells react differently to chitin oligosaccharides and Sinorhizobium meliloti nodulation factors. Planta. 210:157–164. - PubMed
-
- Chen, L.J., Zou, W.S., Fei, C.Y., Wu, G., Li, X.Y., Lin, H.H., et al. (2018) α-Expansin EXPA4 positively regulates abiotic stress tolerance but negatively regulates pathogen resistance in Nicotiana tabacum. Plant Cell Physiol. 59:2317–2330. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

