The role of multivalency in the association of the eight twenty-one protein 2 (ETO2) with the nucleosome remodeling and deacetylase (NuRD) complex
- PMID: 40421803
- PMCID: PMC12107431
- DOI: 10.1093/nar/gkaf439
The role of multivalency in the association of the eight twenty-one protein 2 (ETO2) with the nucleosome remodeling and deacetylase (NuRD) complex
Abstract
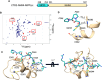
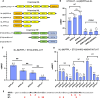
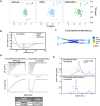
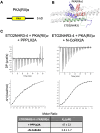
Over the past 50 years, research has uncovered the co-regulatory proteins and complexes that silence the expression of the γ-globin gene in a developmental stage-specific manner. Recent research expanded the list of these regulatory factors by showing that the eight twenty-one protein 2 (ETO2) helps recruit the nucleosome remodeling and deacetylase (NuRD) complex to the globin locus. Furthermore, ETO2 regulates hematopoietic differentiation and is a potential therapeutic target for acute leukemia. In this work, we identify critical interactions between ETO2 and the GATA Zn finger domain containing the 2A (GATAD2A) component of NuRD. The ETO2 nervy homology region 4 (NHR4) domain interacts with multiple polyproline-leucine motifs within GATAD2A. We demonstrate that oligomerization of the ETO2 nervy homology region 3 (NHR3) enhances its affinity for peptides containing at least two polyproline-leucine motifs. Replacing the native motifs from GATAD2A with a higher-affinity sequence from known-binder N-CoR markedly enhances binding affinity, yielding a peptide that disrupts the interaction between ETO2 and target proteins. Enforced peptide expression elevates γ-globin expression levels and induces differentiation of HUDEP-2 and K562 cells. These findings provide insight into ETO2-mediated recruitment of co-regulatory proteins and yield a novel approach for ETO2 inhibition through multivalent binding of the NHR4 domain.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures






References
-
- Hoang T, Lambert JA, Martin R SCL/TAL1 in hematopoiesis and cellular reprogramming. Curr Top Dev Biol. 2016; 118:163–204. - PubMed
MeSH terms
Substances
Grants and funding
- P30CA016059/UNC Lineberger Cancer Center
- P30 CA016086/CA/NCI NIH HHS/United States
- VCU Massey Cancer Center
- R01GM118499/GM/NIGMS NIH HHS/United States
- UNC Eshelman School of Pharmacy
- R35 GM145227/GM/NIGMS NIH HHS/United States
- P30 CA016059/CA/NCI NIH HHS/United States
- R35GM145227/GM/NIGMS NIH HHS/United States
- R01 DK115563/DK/NIDDK NIH HHS/United States
- R56DK29902/National Institute of Diabetes, Digestive, and Kidney Diseases
- R01 GM118499/GM/NIGMS NIH HHS/United States
- R56 DK029902/DK/NIDDK NIH HHS/United States
- UNC Lineberger Comprehensive Cancer Center
- P30CA16086/CA/NCI NIH HHS/United States
- R01DK115563/National Institute of Diabetes, Digestive, and Kidney Diseases
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

