Efficacy and safety of surufatinib in the treatment of thymic neuroendocrine tumors: a 102-case retrospective study
- PMID: 40421954
- PMCID: PMC12107545
- DOI: 10.1093/oncolo/oyaf072
Efficacy and safety of surufatinib in the treatment of thymic neuroendocrine tumors: a 102-case retrospective study
Abstract
Introduction or hypothesis: The SANET-ep study confirmed that patients with non-pancreatic NET could benefit from surufatinib treatment compared with placebo. However, there is a lack of sufficient retrospective data on surufatinib's efficacy in treating thymic neuroendocrine tumors (TNETs). This study aimed to evaluate the efficacy and safety of surufatinib in well-differentiated TNETs by conducting a single center retrospective analysis.
Methods: We conducted a retrospective study at Fudan University Shanghai Cancer Center, including 102 patients with TNETs. This study assessed the efficacy and safety of surufatinib in this population.
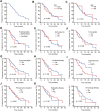
Results: Median progression-free survival was 10.0 months, ORR was 5.6%, and DCR was 83.1%. Surufatinib performed better in first-line treatment compared to secondary treatment in terms of ORR, DCR, and mPFS. The most common adverse events were diarrhea (15.7%), hypertension (19.1%), proteinuria (13.5%), and hypothyroidism (5.6%). About 33.3% of patients required dosage reduction from 300 mg/day to 200 mg/day (or 250 mg/day) due to adverse events, which in most cases were mitigated or disappeared with dose reduction.
Conclusions: For patients with advanced, progressive, well-differentiated TNETs, Surufatinib can be used as an option with a high mPFS and DCR. Moreover, the safety profile of surufatinib was found to be acceptable. These findings suggest that surufatinib could potentially serve as an innovative therapeutic option for this particular patient population.
Keywords: neuroendocrine tumor; surufatinib; thymic; treatment.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): a randomised, double-blind, placebo-controlled, phase 3 study.Lancet Oncol. 2020 Nov;21(11):1489-1499. doi: 10.1016/S1470-2045(20)30493-9. Epub 2020 Sep 20. Lancet Oncol. 2020. PMID: 32966810 Clinical Trial.
-
Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): a randomised, double-blind, placebo-controlled, phase 3 study.Lancet Oncol. 2020 Nov;21(11):1500-1512. doi: 10.1016/S1470-2045(20)30496-4. Epub 2020 Sep 20. Lancet Oncol. 2020. PMID: 32966811 Clinical Trial.
-
Real world study on efficacy and safety of surufatinib in advanced solid tumors evaluation.Sci Rep. 2025 May 10;15(1):16294. doi: 10.1038/s41598-025-00974-8. Sci Rep. 2025. PMID: 40348748 Free PMC article.
-
Surufatinib for the treatment of advanced extrapancreatic neuroendocrine tumors.Expert Rev Anticancer Ther. 2021 Sep;21(9):917-926. doi: 10.1080/14737140.2021.1944110. Epub 2021 Jun 29. Expert Rev Anticancer Ther. 2021. PMID: 34142932
-
Current treatments and future potential of surufatinib in neuroendocrine tumors (NETs).Ther Adv Med Oncol. 2021 Aug 31;13:17588359211042689. doi: 10.1177/17588359211042689. eCollection 2021. Ther Adv Med Oncol. 2021. PMID: 34484432 Free PMC article. Review.
References
-
- Gaur P, Leary C, Yao JC.. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg. 2010;251:1117-1121. https://doi.org/10.1097/SLA.0b013e3181dd4ec4 - DOI - PubMed
-
- Ragnarsson O, Juhlin CC, Torpy DJ, Falhammar H.. A clinical perspective on ectopic Cushing’s syndrome. Trends Endocrinol Metab. 2024;35:347-360. https://doi.org/10.1016/j.tem.2023.12.003 - DOI - PubMed
-
- Simonds WF. Expressions of Cushing’s syndrome in multiple endocrine neoplasia type 1. Front Endocrinol. 2023;14:1183297. https://doi.org/10.3389/fendo.2023.1183297 - DOI - PMC - PubMed
-
- Thompson R, Landry CS.. Multiple endocrine neoplasia 1: a broad overview. Ther Adv Chronic Dis. 2021;12:20406223211035288. https://doi.org/10.1177/20406223211035288 - DOI - PMC - PubMed
-
- Guerrero-Pérez F, Peiró I, Marengo AP, et al.Ectopic Cushing’s syndrome due to thymic neuroendocrine tumours: a systematic review. Rev Endocr Metab Disord. 2021;22:1041-1056. https://doi.org/10.1007/s11154-021-09660-2 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

