Aptamer and Oligonucleotide-Based Biosensors for Health Applications
- PMID: 40422016
- PMCID: PMC12109747
- DOI: 10.3390/bios15050277
Aptamer and Oligonucleotide-Based Biosensors for Health Applications
Abstract
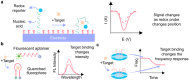
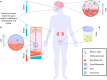
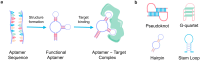
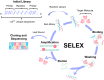
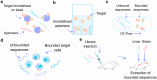
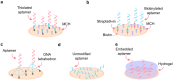
Aptamers have emerged as powerful molecular recognition elements for biosensing applications, offering high specificity, stability, and adaptability. This review explores key considerations in designing aptamer-based sensors (aptasensors), with a focus on biomarker selection, aptamer design, and detection and immobilization strategies. However, challenges such as biofluid stability and reversibility must be addressed to improve biosensor performance. In this study, the potential of aptamer-based platforms in diagnostics is explored, emphasizing their advantages and future applications. Looking ahead, advances in multifunctional aptamers, integration with nanomaterials, and computational optimization are highlighted as promising directions for enhancing their effectiveness in biosensing.
Keywords: aptamer biosensor; electrochemical aptasensor; health monitoring.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Arroyo-Currás N., Dauphin-Ducharme P., Scida K., Chávez J.L. From the Beaker to the Body: Translational Challenges for Electrochemical, Aptamer-Based Sensors. Anal. Methods. 2020;12:1288–1310. doi: 10.1039/D0AY00026D. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

