Anti-Adalimumab Antibodies Purified from Juvenile Idiopathic Arthritis Patients: Kinetic Characterization Among Biosimilars
- PMID: 40422017
- PMCID: PMC12110412
- DOI: 10.3390/bios15050278
Anti-Adalimumab Antibodies Purified from Juvenile Idiopathic Arthritis Patients: Kinetic Characterization Among Biosimilars
Abstract
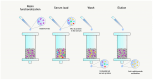
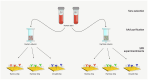
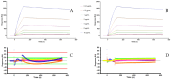
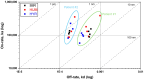
The use of adalimumab biosimilars has become increasingly common in clinical practice, reflecting their growing acceptance and efficacy as therapeutic alternatives to reference biologics. However, studies investigating the molecular interactions between anti-adalimumab antibodies (AAA) elicited in patients and different adalimumab biosimilars remain limited. This study aims to characterize the kinetic interactions between purified AAA from pediatric patients with Juvenile Idiopathic Arthritis and three adalimumab formulations: the originator Humira®, and the biosimilars GP2017 (Hyrimoz®) and SB5 (Imraldi®). For this purpose, adalimumab formulations were immobilized on a gold chip, and purified AAA were flowed to perform further kinetic analysis using the surface plasmon resonance (SPR) technology. Results showed that the KD values for purified AAA from patients treated with biosimilars GP2017 (Hyrimoz®) or SB5 (Imraldi®) were comparable across all formulations tested, including the originator Humira®. AAA interacted with Humira®, Hyrimoz®, and Imraldi® with similar apparent affinity (10-9 M > KD > 10-10 M); slight variations have been observed among patients, less among biosimilars. The similarity in KD values across biosimilars and the originator supports the notion that, at the level of immunogenicity, biosimilars can be considered clinically comparable to the originator.
Keywords: adalimumab; anti-drug antibodies; biosimilars; juvenile idiopathic arthritis; surface plasmon resonance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- GaBI Journal Editor Patent Expiry Dates for Biologicals: 2018 Update. GaBI J. 2019;8:24–31. doi: 10.5639/gabij.2019.0801.003. - DOI
-
- Biosimilar Medicines: Overview | European Medicines Agency (EMA) [(accessed on 7 October 2024)]. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/biosimilar-medici....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

