Naringenin Targets PI3K p85alpha to Suppress PI3K/AKT Signaling Pathway and Ameliorate Disordered MMP-9 Secretion in Cigarette Smoke Extract-Induced Alveolar Macrophages In Vitro
- PMID: 40422181
- PMCID: PMC12109897
- DOI: 10.3390/cells14100678
Naringenin Targets PI3K p85alpha to Suppress PI3K/AKT Signaling Pathway and Ameliorate Disordered MMP-9 Secretion in Cigarette Smoke Extract-Induced Alveolar Macrophages In Vitro
Abstract
Background: Naringenin has demonstrated potential therapeutic effects against cigarette smoke-induced lung injury; however, its underlying mechanisms of regulating matrix metalloproteinase-9 (MMP-9) in alveolar macrophages remain unclear.
Methods: The regulatory mechanisms of naringenin in cigarette smoke extract (CSE)-induced alveolar macrophages were investigated using proteomics, and then, naringenin's targets were further validated by Western blot, molecular docking, molecular dynamics (MD) simulations, cellular thermal shift assay (CETSA), and enzyme activity assay.
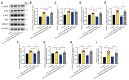
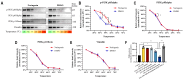
Results: The proteomics revealed that the PI3K/AKT signaling pathway might play a crucial role in naringenin's inhibition of MMP-9. Western blot analysis confirmed that naringenin significantly inhibited CSE-upregulated PI3K/AKT signaling pathway and reduced MMP-9 expression in MH-S cells. Notably, the PI3K activator 740Y-P reversed naringenin's effects on MMP-9. Additionally, molecular docking, MD simulations, and CETSA identified PI3K p85alpha as the potential binding site for naringenin, and naringenin markedly inhibited CSE-induced PI3K activity. In in vitro experiments, naringenin inhibiting MMP-9 secretion in alveolar macrophages contributed to alleviating elastin and E-cadherin damage in alveolar epithelial cells. Furthermore, naringenin effectively suppressed CSE-induced MMP-9 secretion in primary mouse alveolar macrophages and human THP-1-differentiated macrophages.
Conclusions: Our findings revealed that naringenin, a potential candidate for treating smoking-induced lung injury, directly targeted PI3K p85alpha, inhibiting PI3K activity and MMP-9 expression in CSE-induced alveolar macrophages via suppressing the PI3K/AKT signaling pathway.
Keywords: MMP-9; PI3K p85alpha; alveolar macrophage; cigarette; naringenin.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Simvastatin inhibits induction of matrix metalloproteinase-9 in rat alveolar macrophages exposed to cigarette smoke extract.Exp Mol Med. 2009 Apr 30;41(4):277-87. doi: 10.3858/emm.2009.41.4.031. Exp Mol Med. 2009. PMID: 19299917 Free PMC article.
-
Naringenin regulates cigarette smoke extract-induced extracellular vesicles from alveolar macrophage to attenuate the mouse lung epithelial ferroptosis through activating EV miR-23a-3p/ACSL4 axis.Phytomedicine. 2024 Feb;124:155256. doi: 10.1016/j.phymed.2023.155256. Epub 2023 Dec 10. Phytomedicine. 2024. PMID: 38181527
-
MMP-2 and MMP-9 mediate cigarette smoke extract-induced epithelial-mesenchymal transition in airway epithelial cells via EGFR/Akt/GSK3β/β-catenin pathway: Amelioration by fisetin.Chem Biol Interact. 2019 Dec 1;314:108846. doi: 10.1016/j.cbi.2019.108846. Epub 2019 Oct 10. Chem Biol Interact. 2019. PMID: 31606474
-
Effect of simvastatin on MMPs and TIMPs in cigarette smoke-induced rat COPD model.Int J Chron Obstruct Pulmon Dis. 2017 Feb 22;12:717-724. doi: 10.2147/COPD.S110520. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28260878 Free PMC article.
-
Understanding potential mechanisms of harm: the drivers of electronic cigarette-induced changes in alveolar macrophages, neutrophils, and lung epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2021 Aug 1;321(2):L336-L348. doi: 10.1152/ajplung.00081.2021. Epub 2021 May 19. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34009037 Review.
References
-
- Jokipii Krueger C.C., Park S.L., Patel Y., Stram D.O., Aldrich M., Cai Q., Tretyakova N.Y. Association of Urinary N7-(1-hydroxyl-3-buten-1-yl) Guanine (EB-GII) Adducts and Butadiene-Mercapturic Acids with Lung Cancer Development in Cigarette Smokers. Chem. Res. Toxicol. 2024;37:374–384. doi: 10.1021/acs.chemrestox.3c00336. - DOI - PMC - PubMed
-
- Agache I., Ricci-Cabello I., Canelo-Aybar C., Annesi-Maesano I., Cecchi L., Biagioni B., Chung K.F., D’Amato G., Damialis A., Del Giacco S., et al. The impact of exposure to tobacco smoke and e-cigarettes on asthma-related outcomes: Systematic review informing the EAACI guidelines on environmental science for allergic diseases and asthma. Allergy. 2024;79:2346–2365. doi: 10.1111/all.16151. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2023A1515011953/the Natural Science Foundation of Guangdong Province
- 2022A1515012228/the Guangdong Basic and Applied Basic Research Foundation
- 2022SDZG07/the Open Competition Program of Ten Major Directions of Agricultural Science and Technology Innovation for the 14th Five Year Plan of Guangdong Province
- 2022ZD006/the Research Fund of Maoming Branch, Guangdong Laboratory for Lingnan Modern Agriculture
LinkOut - more resources
Full Text Sources
Miscellaneous

