The Identification of Opioid Receptors and Peptide Precursors in Human DRG Neurons Expressing Pain-Signaling Molecules Confirms Their Potential as Analgesic Targets
- PMID: 40422197
- PMCID: PMC12110618
- DOI: 10.3390/cells14100694
The Identification of Opioid Receptors and Peptide Precursors in Human DRG Neurons Expressing Pain-Signaling Molecules Confirms Their Potential as Analgesic Targets
Abstract
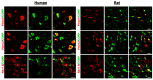
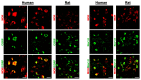
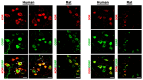
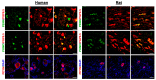
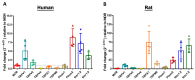
The presence and function of the opioidergic system in sensory dorsal root ganglia (DRG) was demonstrated in various animal models of pain. To endorse recent functional and transcriptional evidence of opioid receptors in human DRG, this study compared morphological and transcriptional evidence in human and rat DRG using immunofluorescence confocal microscopy and mRNA transcript analysis. Specifically, it examined the neuronal expression of mu (MOR), delta (DOR), and kappa (KOR) opioid receptors, opioid peptide precursors (POMC, PENK, and PDYN), and key pain-signaling molecules. The results demonstrate abundant immunoreactivity in human DRG for key pain transduction receptors, including the thermosensitive ion channels TRPV1, TRPV4 and TRPA1, mechanosensitive PIEZO1 and PIEZO2, and the nociceptive-specific Nav1.8. They colocalized with calcitonin gene-related peptide (CGRP), a marker for peptidergic sensory neurons. Within this same subpopulation, we identified MOR, DOR, and KOR, while their ligand precursors were less abundant. Notably, the mRNA transcripts of MOR and PENK in human DRG were highest among the opioid-related genes; however, they were considerably lower than those of key pain-signaling molecules. These findings were corroborated by functional evidence in demonstrating the fentanyl-induced inhibition of voltage-gated calcium currents in rat DRG, which was antagonized by naloxone. The immunohistochemical and transcriptional demonstration of opioid receptors and their endogenous ligands in both human and rat DRG support recent electrophysiologic and in situ hybridization evidence in human DRG and confirms their potential as analgesic targets. This peripherally targeted approach has the advantage of mitigating central opioid-related side effects, endorsing the potential of future translational pain research from rodent models to humans.
Keywords: human; opioid peptides; opioid receptors; pain; peripheral sensory neurons.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

