Self-reported oral health outcomes after switching to a novel nicotine pouch technology: a pilot study
- PMID: 40422555
- PMCID: PMC12138386
- DOI: 10.2340/aos.v84.43805
Self-reported oral health outcomes after switching to a novel nicotine pouch technology: a pilot study
Abstract
Objective: Nicotine pouch use has been linked to oral health concerns, including oral lesions and gingival irritation. This pilot study examines self-reported oral health outcomes following the use of a novel nicotine pouch (Stingfree Strong Blue Mint), with an impermeable barrier on the interior side designed to reduce mucosal irritation.
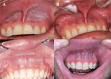
Materials and methods: A total of 23 Swedish dentists who were current snus or nicotine pouch users participated in a 5-week observational study. Baseline and follow-up assessments included self-reported oral health status and photographic documentation of mucosal conditions, reviewed by an independent blinded dental expert. Primary outcomes included changes in self-reported snus lesions, gingival recession, gingival irritation, and gingivitis. Results: The prevalence of self-reported snus lesions decreased from 95.7% (n = 22) to 69.6% (n = 16). Median Axell-scale lesion severity declined from 2 (interquartile range [IQR]: 1-3) to 1 (IQR: 0-2) (z = 3.756, p = 0.0002). Moderate-to-severe lesions (Axell score ≥ 3) dropped from 39.1% (n = 9) to 0% (n = 0). Self-reported gingivitis cases (n = 3) were eliminated, and gingival irritation decreased by 90.0%.
Conclusions: Preliminary findings suggest that the use of the Stingfree Strong Blue Mint nicotine pouch may reduce mucosal irritation. While promising, these findings warrant validation through large randomised controlled trials to establish long-term effectiveness and safety.
Conflict of interest statement
GRMLR, SAP, JK, RG, SG, GB, VF, and AA declare no conflict of interest.
IC provides consulting advice to various oral healthcare companies, including Unilever, Philips, Haleon, P&G, Johnson & Johnson.
KF has received consulting fees from many companies that develop or market pharmaceutical and behavioural treatments for smoking cessation. In the year 2000, he started a company Niconovum that developed the first non-tobacco nicotine pouch that was licensed as an NRT. He currently receives consulting fees from Swedish Match and has received fees in the past from tobacco companies to assist their development of less-risky tobacco products.
RP is full tenured professor of Internal Medicine at the University of Catania (Italy) and Medical Director of the Institute for Internal Medicine and Clinical Immunology at the same University. He has received grants from U-BIOPRED and AIR-PROM, Integral Rheumatology & Immunology Specialists Network (IRIS), Global Action to End Smoking (previously known as ‘Foundation for a Smoke Free World’), Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, Merk Sharp & Dohme, Boehringer Ingelheim, Novartis, Arbi Group Srl., Duska Therapeutics, Forest Laboratories and Ministero dell Universita’ e della Ricerca (MUR) Bando PNRR 3277/2021 (CUP E63C22000900006) and 341/2022 (CUP E63C22002080006), funded by NextGenerationEU, the European Union (EU) economic recovery package. He is founder of the Center for Tobacco Prevention and Treatment (CPCT) at the University of Catania and of the Center of Excellence for the Acceleration of Harm Reduction at the same university. He receives consultancy fees from Pfizer, Boehringer Ingelheim, Duska Therapeutics, Forest Laboratories, CV Therapeutics, and Sermo Inc. He is being paid textbook royalties from Elsevier. He is also involved in a patent application for ECLAT Srl. He is a pro bono scientific advisor for Lega Italiana Anti Fumo (LIAF) and the International Network of Nicotine Consumers Organizations (INNCO); and he is Chair of the European Technical Committee for Standardization on ‘Requirements and test methods for emissions of electronic cigarettes’ (CEN/TC 437; WG4).
Figures
Similar articles
-
The effect of a non-tobacco-based nicotine pouch on mucosal lesions caused by Swedish smokeless tobacco (snus).Eur J Oral Sci. 2022 Aug;130(4):e12885. doi: 10.1111/eos.12885. Epub 2022 Jul 19. Eur J Oral Sci. 2022. PMID: 35853092 Free PMC article.
-
Reduction in nicotine intake and oral mucosal changes among users of Swedish oral moist snuff after switching to a low-nicotine product.J Oral Pathol Med. 1995 Jul;24(6):244-50. doi: 10.1111/j.1600-0714.1995.tb01176.x. J Oral Pathol Med. 1995. PMID: 7562659
-
Acute effects of snus in never-tobacco users: a pilot study.Am J Drug Alcohol Abuse. 2018;44(1):113-119. doi: 10.1080/00952990.2016.1260581. Epub 2016 Dec 8. Am J Drug Alcohol Abuse. 2018. PMID: 27929684 Free PMC article. Clinical Trial.
-
Home use of interdental cleaning devices, in addition to toothbrushing, for preventing and controlling periodontal diseases and dental caries.Cochrane Database Syst Rev. 2019 Apr 10;4(4):CD012018. doi: 10.1002/14651858.CD012018.pub2. Cochrane Database Syst Rev. 2019. PMID: 30968949 Free PMC article.
-
Health Effects and Dependency Associated with Snuff Consumption [Internet].Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2005 Jun. Report from Norwegian Knowledge Centre for the Health Services (NOKC) No. 06-2005. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2005 Jun. Report from Norwegian Knowledge Centre for the Health Services (NOKC) No. 06-2005. PMID: 29319988 Free Books & Documents. Review.
References
-
- Rodgman A, Perfetti TA. The chemical components of tobacco and tobacco smoke. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2009.
-
- U.S. Department of Health and Human Services . The health consequences of smoking: 50 years of progress: a report of the surgeon general. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
-
- U.S. Department of Health and Human Services . How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the surgeon general. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. - PubMed
-
- Azzopardi D, Haswell LE, Frosina J, McEwan M, Gale N, Thissen J, et al. . Assessment of biomarkers of exposure and potential harm, and physiological and subjective health measures in exclusive users of nicotine pouches and current, former and never smokers. Biomarkers. 2023 Feb;28(1):118–29. 10.1080/1354750X.2022.2148747 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

