Anticancer Mechanisms of Ginsenoside Compound K: A Review
- PMID: 40422575
- PMCID: PMC12110123
- DOI: 10.3390/diseases13050143
Anticancer Mechanisms of Ginsenoside Compound K: A Review
Abstract
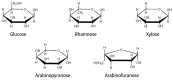
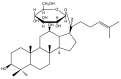
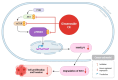
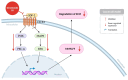
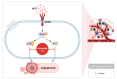
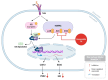
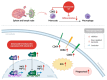
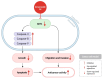
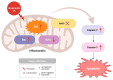
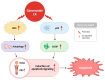
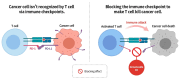
Cancer, also known as malignant tumors, is formed due to abnormal mutations and the proliferation of human cells. Cancer cells not only demonstrate accelerated proliferation but also show robust invasive and metastatic potential, disseminating from a primary affected region of the body to multiple areas and potentially culminating in organ dysfunction or failure, thereby jeopardizing the individual's life. The rapid growth of the biopharmaceutical market has given rise to numerous novel medicines, thereby precipitating a paradigm shift in contemporary drug development methodologies. This modification is focused on identifying methodologies that can effectively target cancerous cells while minimizing damage to normal cells. There is an increasing societal movement that supports the utilization of natural ingredients derived from plants. In recent years, traditional herbal medicine has experienced a surge in popularity within the global cancer market. In comparison with the use of more toxic chemotherapy methods, there has been an increasing focus on advanced therapies that exhibit reduced side effects. Ginsenoside compound K (CK) is derived from the natural components in ginseng through biotransformation. The utilization of CK in cancer research is a practice engaged in by numerous scientists. The underlying rationale is that CK exhibits a multitude of effects within the realm of cancer research, including but not limited to the mitigation of inflammation, the suppression of cancerous cell proliferation, and the safeguarding of cardiovascular, hepatic, and renal functions. This review methodically identifies and organizes CK-related journals according to the following key points of cancer treatment: the effects on cancer cells themselves, angiogenesis inhibition, modulation of immune response to identify cancer cells, and inflammation regulation. The intricate interplay between ginsenoside CK and cells is elucidated through a graphical representation. The present review focuses on the results of CK in in vitro tests. It is our hope that the present article will aid future studies on the results of CK in vivo tests, clarify the correlation between cellular mechanisms in vivo and in vitro tests, and assist in the development of drugs.
Keywords: angiogenesis; cancer; chronic inflammation; ginsenoside compound K; stromal cell-derived factor 1.
Conflict of interest statement
Sheau-Long Lee is the owner of Wellhead Biological Technology Corp., Taiwan. Hui-Ting Chan, Tzu-Hsuan Li and Yu-Quan Chang is an employee of Wellhead Biological Technology company. Hui-Ting Chan is also a PhD student at the Biotechnology Industry Master and Ph.D. Program in Chang Gung University, Taiwan. The authors declare no conflicts of interest. The Wellhead biological technology company engaged in a collaborative effort with Professor Robert Y L Wang to elucidate the anticancer mechanisms of ginsenoside compound K against various cancer cells.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

