Development and Investigation of a New Polysulfone Dialyzer with Increased Membrane Hydrophilicity
- PMID: 40422742
- PMCID: PMC12113146
- DOI: 10.3390/membranes15050132
Development and Investigation of a New Polysulfone Dialyzer with Increased Membrane Hydrophilicity
Abstract
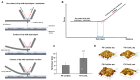
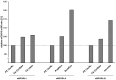
Innovation in dialysis care is fundamental to improve well-being and outcomes of patients with end-stage kidney disease. The dialyzer is the core element of dialysis treatments, as it largely defines which substances are removed from the patient's body. Moreover, its large surface size is the major place of interaction of the patient's blood with artificial surfaces and thus may lead to undesired effects such as inflammation or coagulation. In the present article we summarize the development path for a new dialyzer, including in vitro and clinical evidence generation. We use the example of the novel FX CorAL dialyzer, which has recently entered European and US markets, to show which steps are needed to develop and characterize a new dialyzer. The FX CorAL dialyzer includes a new hydrophilic membrane, which features reduced protein adsorption, sustained performance, and an improved hemocompatibility profile, characterized in numerous in vitro and clinical studies. Safety evaluations revealed a favorable profile, with low incidences of adverse device effects. Insights gained from both in vitro and clinical studies contribute to the advancement of dialyzer development, ultimately leading to improved patient care.
Keywords: dialyzer; end stage kidney disease; hemocompatibility; hydrophilicity; membrane; performance.
Conflict of interest statement
A.M.Z., B.G, A.E., S.B., N.C., M.S.-G., J.P.K. and T.L. are employees of Fresenius Medical Care and may hold stock in the company. B.O. reports consulting fees from Fresenius Medical Care.
Figures


References
-
- Ronco C., Bowry S.K., Brendolan A., Crepaldi C., Soffiati G., Fortunato A., Bordoni V., Granziero A., Torsello G., La Greca G. Hemodialyzer: From Macro-Design to Membrane Nanostructure; the Case of the FX-Class of Hemodialyzers. Kidney Int. Suppl. 2002;61:S126–S142. doi: 10.1046/j.1523-1755.61.s80.23.x. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

