Improvement of Catalytic Activity and Thermostability of Alginate Lyase VxAly7B-CM via Rational Computational Design Strategies
- PMID: 40422788
- PMCID: PMC12112969
- DOI: 10.3390/md23050198
Improvement of Catalytic Activity and Thermostability of Alginate Lyase VxAly7B-CM via Rational Computational Design Strategies
Abstract
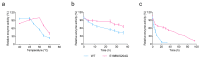
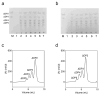
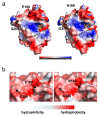
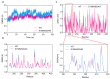
Alginate lyase degrades alginate through the β-elimination mechanism to produce alginate oligosaccharides (AOS) with notable biochemical properties and diverse biological activities. However, its poor thermostability limits large-scale industrial production. In this study, we employed a rational computational design strategy combining computer-aided evolutionary coupling analysis and ΔΔGfold evaluation to enhance both the thermostability and catalytic activity of the alginate lyase VxAly7B-CM. Among ten single-point mutants, the E188N and S204G mutants exhibited increases in Tm from 47.0 °C to 48.9 °C and 50.2 °C, respectively, with specific activities of 3701.02 U/mg and 2812.01 U/mg at 45 °C. Notably, the combinatorial mutant E188N/S204G demonstrated a ΔTm of 5 °C and an optimal reaction temperature up to 50 °C, where its specific activity reached 3823.80 U/mg-a 31% increase. Moreover, its half-life at 50 °C was 38.4 h, which is 7.0 times that of the wild-type enzyme. Protein structural analysis and molecular dynamics simulations suggested that the enhanced catalytic performance and thermostability of the E188N/S204G mutant may be attributed to optimized surface charge distribution, strengthened hydrophobic interactions, and increased tertiary structure stability. Overall, our findings provided valuable insights into enzyme stabilization strategies and supported the industrial production of functional AOS.
Keywords: alginate lyase; alginate oligosaccharides; molecular dynamics simulation; rational design; thermostability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Improving the thermostability of a novel PL-6 family alginate lyase by rational design engineering for industrial preparation of alginate oligosaccharides.Int J Biol Macromol. 2023 Sep 30;249:125998. doi: 10.1016/j.ijbiomac.2023.125998. Epub 2023 Jul 26. Int J Biol Macromol. 2023. PMID: 37499708
-
Computer-Aided Rational Design Strategy to Improve the Thermal Stability of Alginate Lyase AlyMc.J Agric Food Chem. 2024 Feb 14;72(6):3055-3065. doi: 10.1021/acs.jafc.3c07215. Epub 2024 Feb 1. J Agric Food Chem. 2024. PMID: 38298105
-
Improving thermostability of a PL 5 family alginate lyase with combination of rational design strategies.Int J Biol Macromol. 2023 Jul 1;242(Pt 2):124871. doi: 10.1016/j.ijbiomac.2023.124871. Epub 2023 May 16. Int J Biol Macromol. 2023. PMID: 37201879
-
Molecular Engineering of Alginate Lyases and the Potential Agricultural Applications of Their Enzymatic Products.J Agric Food Chem. 2025 Mar 12;73(10):5666-5684. doi: 10.1021/acs.jafc.4c09913. Epub 2025 Feb 26. J Agric Food Chem. 2025. PMID: 40011194 Review.
-
Advances in alginate lyases and the potential application of enzymatic prepared alginate oligosaccharides: A mini review.Int J Biol Macromol. 2024 Mar;260(Pt 1):129506. doi: 10.1016/j.ijbiomac.2024.129506. Epub 2024 Jan 18. Int J Biol Macromol. 2024. PMID: 38244735 Review.
References
-
- Pritchard M.F., Powell L.C., Jack A.A., Powell K., Beck K., Florance H., Forton J., Rye P.D., Dessen A., Hill K.E., et al. A Low-Molecular-Weight Alginate Oligosaccharide Disrupts Pseudomonal Microcolony Formation and Enhances Antibiotic Effectiveness. Antimicrob. Agents Chemother. 2017;61:e00762-17. doi: 10.1128/AAC.00762-17. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2023CXGC010414/Shandong Province Key R&D Program
- 2024YFC2816005/National Key R&D Program of China
- 2022QNLM030003-1/Qingdao Marine Science and Technology Center
- 2018YFC0311105/Shandong Province Technology Innovation Guidance Program
- 22-3-6-gjxm-5-gx, 21-1-6-gjxm-27-gx and 20-11-6-64-gx/Science & Technology Development Project of Qingdao
LinkOut - more resources
Full Text Sources

