Protective Group-Dependent Iridium-Catalyzed CH Borylations of Levodopa
- PMID: 40422969
- PMCID: PMC12150333
- DOI: 10.1021/acs.joc.5c00476
Protective Group-Dependent Iridium-Catalyzed CH Borylations of Levodopa
Abstract
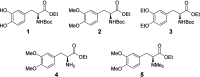
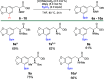
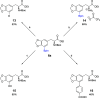
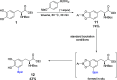
Despite being an important aromatic amino acid, iridium-catalyzed borylation (CHB) of levodopa has not been reported. Via the application of carefully chosen protecting groups for the catechol moiety, the steric hindrance around the arene can be reduced, enabling selective C5 CHB of levodopa. Methylene and boronic ester protecting groups were explored, the latter of which is deprotected in situ to yield the free catechol.
Figures
References
-
- Mossine A. V., Tanzey S. S., Brooks A. F., Makaravage K. J., Ichiishi N., Miller J. M., Henderson B. D., Erhard T., Bruetting C., Skaddan M. B., Sanford M. S., Scott P. J. H.. Synthesis of High-Molar-Activity [18F]6-Fluoro-l-DOPA Suitable for Human Use via Cu-Mediated Fluorination of a BPin Precursor. Nat. Protoc. 2020;15(5):1742–1759. doi: 10.1038/s41596-020-0305-9. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

