Methodologies for the Emulation of Biomarker-Guided Trials Using Observational Data: A Systematic Review
- PMID: 40423066
- PMCID: PMC12112824
- DOI: 10.3390/jpm15050195
Methodologies for the Emulation of Biomarker-Guided Trials Using Observational Data: A Systematic Review
Abstract
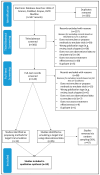
Background: Target trial emulation involves the application of design principles from randomised controlled trials (RCTs) to observational data, and is particularly useful in situations where an RCT would be unfeasible. Biomarker-guided trials, which incorporate biomarkers within their design to either guide treatment and/or determine eligibility, are often unfeasible in practice due to sample size requirements or ethical concerns. Here, we undertake a systematic review of methodologies used in target trial emulations, comparing treatment effectiveness, critically appraising them, and considering their applicability to the emulation of biomarker-guided trials. Methods: A comprehensive search strategy was developed to identify studies reporting on methods for target trial emulation comparing the effectiveness of treatments using observational data, and applied to the following bibliographic databases: PubMed, Scopus, Web of Science, and Ovid MEDLINE. A narrative description of methods identified in the review was undertaken alongside a critique of their relative strengths and limitations. Results: We identified a total of 59 papers: 47 emulating a target trial ('application' studies), and 12 detailing methods to emulate a target trial ('methods' studies). A total of 25 papers were identified as emulating a biomarker-guided trial (42%). While all papers reported methods to adjust for baseline confounding, 40% of application papers did not specify methods to adjust for time-varying confounding. Conclusions: This systematic review has identified a range of methods used to control for baseline, time-varying, and residual/unmeasured confounding within target trial emulation and provides a guide for researchers interested in emulation of biomarker-guided trials.
Keywords: biomarker-guided trials; personalised medicine; target trial emulation.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Conducting observational analyses with the target trial emulation approach: a methodological systematic review.BMJ Open. 2024 Nov 12;14(11):e086595. doi: 10.1136/bmjopen-2024-086595. BMJ Open. 2024. PMID: 39532374 Free PMC article.
-
The R.O.A.D. to clinical trial emulation.Res Sq [Preprint]. 2025 Mar 19:rs.3.rs-5576146. doi: 10.21203/rs.3.rs-5576146/v1. Res Sq. 2025. PMID: 40166010 Free PMC article. Preprint.
-
Current practices in studies applying the target trial emulation framework: a protocol for a systematic review.BMJ Open. 2023 Jun 27;13(6):e070963. doi: 10.1136/bmjopen-2022-070963. BMJ Open. 2023. PMID: 37369393 Free PMC article.
-
Emulating Clinical Trials with the Mayo Clinic Platform: Cardiovascular Research Perspective.medRxiv [Preprint]. 2025 Mar 24:2025.03.19.25324271. doi: 10.1101/2025.03.19.25324271. medRxiv. 2025. PMID: 40166580 Free PMC article. Preprint.
References
-
- Brown L.C., Jorgensen A.L., Antoniou M., Wason J. Biomarker-Guided Trials. In: Piantadosi S., Meinert C.L., editors. Principles and Practice of Clinical Trials. Springer International Publishing; Cham, Switzerland: 2022. pp. 1145–1170.
-
- Le Tourneau C., Delord J.-P., Gonçalves A., Gavoille C., Dubot C., Isambert N., Campone C., Trédan O., Massiani M.-A., Mauborgne C., et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16:1324–1334. doi: 10.1016/S1470-2045(15)00188-6. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

