Yeast-Produced Human Recombinant Lysosomal β-Hexosaminidase Efficiently Rescues GM2 Ganglioside Accumulation in Tay-Sachs Disease
- PMID: 40423067
- PMCID: PMC12113087
- DOI: 10.3390/jpm15050196
Yeast-Produced Human Recombinant Lysosomal β-Hexosaminidase Efficiently Rescues GM2 Ganglioside Accumulation in Tay-Sachs Disease
Abstract
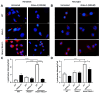
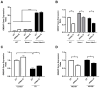
Background: Tay-Sachs disease (TSD) is an autosomal recessive lysosomal storage disorder characterized by the accumulation of GM2 ganglioside due to mutations in the HEXA gene, which encodes the α-subunit of β-Hexosaminidase A. This accumulation leads to significant neuropathological effects and premature death in affected individuals. No effective treatments exist, but enzyme replacement therapies are under investigation. In our previous work, we demonstrated the internalization and efficacy of human recombinant lysosomal β-hexosaminidase A (rhHex-A), produced in the methylotrophic yeast Pichia pastoris, in reducing lipids and lysosomal mass levels in fibroblasts and neural stem cells derived from patient-induced pluripotent stem cells (iPSCs). In this study, we further evaluated the potential of rhHex-A to prevent GM2 accumulation using fibroblast and neuroglia cells from a TSD patient alongside a relevant mouse model. Methods: Fibroblasts and neuroglial cell lines derived from a murine model and TSD patients were treated with 100 nM rhHexA for 72 h. After treatment, cells were stained by anti-GM2 (targeting GM2 ganglioside; KM966) and anti-LAMP1 (lysosomal-associated membrane protein 1) colocalization staining and incubated with 50 nM LysoTracker Red DND-99 to label lysosomes. In addition, GM2AP and HEXB expression were analyzed to assess whether rhHex-A treatment affected the levels of enzymes involved in GM2 ganglioside degradation. Results: Immunofluorescence staining for LysoTracker and colocalization studies of GM2 and Lamp1 indicated reduced lysosomal mass and GM2 levels. Notably, rhHex-A treatment also affected the expression of the HEXB gene, which is involved in GM2 ganglioside metabolism, highlighting a potential regulatory interaction within the metabolic pathway. Conclusions: Here, we report that rhHex-A produced in yeast can efficiently degrade GM2 ganglioside and rescue lysosomal accumulation in TSD cells.
Keywords: GM2; P. pastoris; Tay–Sachs; murine model; rhHex-A.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Progranulin associates with hexosaminidase A and ameliorates GM2 ganglioside accumulation and lysosomal storage in Tay-Sachs disease.J Mol Med (Berl). 2018 Dec;96(12):1359-1373. doi: 10.1007/s00109-018-1703-0. Epub 2018 Oct 20. J Mol Med (Berl). 2018. PMID: 30341570 Free PMC article.
-
Human recombinant lysosomal β-Hexosaminidases produced in Pichia pastoris efficiently reduced lipid accumulation in Tay-Sachs fibroblasts.Am J Med Genet C Semin Med Genet. 2020 Dec;184(4):885-895. doi: 10.1002/ajmg.c.31849. Epub 2020 Oct 27. Am J Med Genet C Semin Med Genet. 2020. PMID: 33111489 Free PMC article.
-
Neural stem cells for disease modeling and evaluation of therapeutics for Tay-Sachs disease.Orphanet J Rare Dis. 2018 Sep 17;13(1):152. doi: 10.1186/s13023-018-0886-3. Orphanet J Rare Dis. 2018. PMID: 30220252 Free PMC article.
-
[Molecular pathogenesis and therapeutic approach of GM2 gangliosidosis].Yakugaku Zasshi. 2013;133(2):269-74. doi: 10.1248/yakushi.12-00199. Yakugaku Zasshi. 2013. PMID: 23370522 Review. Japanese.
-
New Approaches to Tay-Sachs Disease Therapy.Front Physiol. 2018 Nov 20;9:1663. doi: 10.3389/fphys.2018.01663. eCollection 2018. Front Physiol. 2018. PMID: 30524313 Free PMC article. Review.
References
-
- Beck M., Clarke J.T.R., Sandhoff K. The Gangliosidoses. In: Mehta A.B., Winchester B., editors. Lysosomal Storage Disorders. John Wiley & Sons; Hoboken, NJ, USA: 2022. - DOI
-
- Yuziuk J.A., Bertoni C., Beccari T., Orlacchio A., Wu Y.Y., Li S.C., Li Y.T. Specificity of mouse GM2 activator protein and beta-N-acetylhexosaminidases A and B. Similarities and differences with their human counterparts in the catabolism of GM2. J. Biol. Chem. 1998;273:66–72. doi: 10.1074/jbc.273.1.66. - DOI - PubMed
-
- Seyrantepe V., Demir S.A., Timur Z.K., Von Gerichten J., Marsching C., Erdemli E., Oztas E., Takahashi K., Yamaguchi K., Ates N., et al. Murine Sialidase Neu3 facilitates GM2 degradation and bypass in mouse models of Tay-Sachs disease. Exp. Neurol. 2018;299 Pt A:26–41. doi: 10.1016/j.expneurol.2017.09.012. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

