Rose Bengal-Chitosan Nanocomposites for Oral Administration
- PMID: 40423096
- PMCID: PMC12113823
- DOI: 10.3390/nano15100706
Rose Bengal-Chitosan Nanocomposites for Oral Administration
Abstract
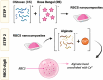
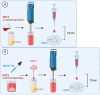
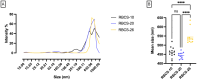
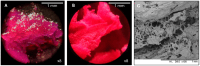
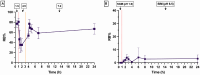
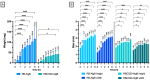
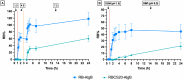
Rose Bengal (RB) holds promise for therapeutic applications in the gastrointestinal (GI) tract but faces significant limitations due to poor bioavailability and stability in the GI environment. This in vitro proof-of-concept study aimed to develop an oral drug delivery system using self-assembled RB-chitosan (RBCS) nanocomposites formed via electrostatic interactions. RBCS nanocomposites exhibited high drug loading efficiency (87%) and a uniform particle size (~443 nm), with physicochemical analyses confirming molecular interactions and structural stability. However, in vitro studies revealed poor and highly variable drug release in simulated gastric fluids (SGFs), underlining the need for further optimization. To address these limitations, RBCS nanocomposites were encapsulated within well-established alginate beads (AlgBs). Among the tested systems, RBCS20-AlgBs were selected as the optimal one, forming a gastroresistant platform. Encapsulation mitigated burst release, enhanced structural integrity, and enabled sustained RB release under intestinal conditions. Swelling studies demonstrated that RBCS20-AlgBs maintained controlled hydration, preventing premature disintegration. Mathematical modeling indicated a matrix relaxation-driven release mechanism, with RBCS20-AlgBs demonstrating improved reproducibility compared to RB-loaded AlgBs (RB-AlgBs). Future studies should focus on evaluating in vivo performance to confirm the system's efficacy for oral administration.
Keywords: Rose Bengal; chitosan; gastrointestinal release; nanocomposites; oral administration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


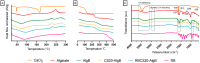
Similar articles
-
Evaluating the efficacy of Rose Bengal-PVA combinations within PCL/PLA implants for sustained cancer treatment.Drug Deliv Transl Res. 2025 May;15(5):1770-1785. doi: 10.1007/s13346-024-01711-w. Epub 2024 Sep 23. Drug Deliv Transl Res. 2025. PMID: 39313735 Free PMC article.
-
Oral delivery of insulin from alginate/chitosan crosslinked by glutaraldehyde.Int J Biol Macromol. 2013 Jul;58:160-8. doi: 10.1016/j.ijbiomac.2013.03.064. Epub 2013 Apr 6. Int J Biol Macromol. 2013. PMID: 23567292
-
Chitosan coating of zein-carboxymethylated short-chain amylose nanocomposites improves oral bioavailability of insulin in vitro and in vivo.J Control Release. 2019 Nov 10;313:1-13. doi: 10.1016/j.jconrel.2019.10.006. Epub 2019 Oct 14. J Control Release. 2019. PMID: 31622690
-
Enhancing Stability and Mucoadhesive Properties of Chitosan Nanoparticles by Surface Modification with Sodium Alginate and Polyethylene Glycol for Potential Oral Mucosa Vaccine Delivery.Mar Drugs. 2022 Feb 22;20(3):156. doi: 10.3390/md20030156. Mar Drugs. 2022. PMID: 35323455 Free PMC article.
-
Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: in vitro and in vivo assessment.Int J Pharm. 2013 Nov 1;456(1):31-40. doi: 10.1016/j.ijpharm.2013.08.037. Epub 2013 Aug 29. Int J Pharm. 2013. PMID: 23994363
References
-
- Kurosu M., Mitachi K., Yang J., Pershing E.V., Horowitz B.D., Wachter E.A., Lacey J.W., Ji Y., Rodrigues D.J. Antibacterial Activity of Pharmaceutical-Grade Rose Bengal: An Application of a Synthetic Dye in Antibacterial Therapies. Molecules. 2022;27:322. doi: 10.3390/molecules27010322. - DOI - PMC - PubMed
-
- Turrini E., Ulfo L., Costantini P.E., Saporetti R., Di Giosia M., Nigro M., Petrosino A., Pappagallo L., Kaltenbrunner A., Cantelli A., et al. Molecular Engineering of a Spheroid-Penetrating Phage Nanovector for Photodynamic Treatment of Colon Cancer Cells. Cell. Mol. Life Sci. 2024;81:144. doi: 10.1007/s00018-024-05174-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

