Phase 1 clinical trial of eneboparatide, a novel PTH receptor 1 agonist
- PMID: 40423237
- PMCID: PMC12186298
- DOI: 10.1530/EC-24-0464
Phase 1 clinical trial of eneboparatide, a novel PTH receptor 1 agonist
Abstract
Objective: This study evaluated the safety, tolerability, pharmacodynamics (PD) and pharmacokinetics (PK) of eneboparatide (AZP-3601), a novel agonist of the PTH receptor 1 developed for the treatment of hypoparathyroidism.
Design: This was a randomized, double-blind, placebo-controlled study. One-hundred four healthy volunteers were recruited into seven single ascending dose (SAD) and five multiple ascending dose (MAD) cohorts.
Methods: PK parameters were time to peak, Cmax, area under the curve (AUC) and half-life. PD parameters included albumin-adjusted serum calcium (sCa), serum phosphorus (sPh), serum endogenous PTH, 24 hr urinary excretion of calcium (24 h-uCa), fractional excretion of calcium (FECa) and bone turnover markers (s-CTX and P1NP).
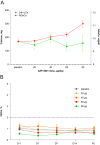
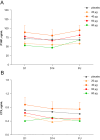
Results: There were no serious adverse events. All adverse events were of mild-to-moderate intensity. AUC and Cmax of eneboparatide increased with increasing doses. Time to maximum plasma concentration was 5-20 min. SAD showed a dose-dependent increase of sCa and decrease of sPh associated with a reduction of serum endogenous PTH. MAD demonstrated a rapid access to maximal PD effects and maintained levels of sCa throughout the day. Urinary excretion of calcium did not increase as a function of the dose of eneboparatide. P1NP and s-CTX did not change over the treatment period.
Conclusion: The PD effects of eneboparatide were prolonged despite the short half-life. These data suggest that eneboparatide may provide sustained control of serum calcium in patients with hypoparathyroidism with once daily dosing. An open-label phase 2 study in patients with hypoparathyroidism has been recently completed and published and a phase 3 study has been initiated.
Clinical trial registration number: NCT05239221.
Keywords: bone; calcium; hypoparathyroidism; kidney; parathormone.
Conflict of interest statement
M Ovize, S Allas, M D Culler, S Milano, T Ouldrouis and M Sumeray were employees of AMOLYT Pharma. J van de Wetering de Rooij and M Mannstadt declare no conflicts of interest.
Figures



References
-
- Vokes T, Rubin MR, Winer KK, et al. Hypoparathyroidism. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 9th edn. Washington, DC, USA: American Society for Bone and Mineral Research. ( 10.1002/9781119266594.ch86) - DOI
-
- Mannstadt M, Bilezikian JP, Thakker RV, et al. Hypoparathyroidism. Nat Rev 2017. 3 1–20. ( 10.1038/nrdp.2017.55) - DOI
Associated data
LinkOut - more resources
Full Text Sources
Medical

