Botulinum Toxin in Pain-Related Post-Stroke Limb Spasticity: A Meta-Analysis of Early and Late Injections
- PMID: 40423340
- PMCID: PMC12116153
- DOI: 10.3390/toxins17050258
Botulinum Toxin in Pain-Related Post-Stroke Limb Spasticity: A Meta-Analysis of Early and Late Injections
Abstract
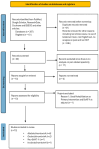
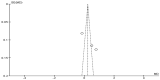
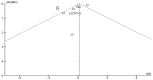
Spasticity is a common complication associated with stroke, and around 72% of stroke patients will develop pain during the disease. Botulinum toxin (BoNT) is a safe and efficacious treatment for spasticity and can improve associated complications, including pain. Hence, this meta-analysis aims to establish whether BoNT can reduce pain-related post-stroke spasticity (pPSS) in the early treatment period (<12 weeks post-stroke) or in the late period (>12 weeks post-stroke) based on the available evidence. This study also aims to establish the dose-response relationship of BoNT-A in pPSS. Based on pooled data from multiple studies, there is no significant difference in the scores measuring pPSS between patients who received early BoNT-A injections and those who received a placebo. This finding suggests that within the early treatment period, BoNT-A may not be more effective than a placebo in reducing pPSS. However, it is important to note that the data for early BoNT-A injections are limited, indicating that research is needed to draw definitive conclusions [z = 3.90 (p < 0.0001)]. While BoNT-A appears somewhat more effective than a placebo in the late phase, as indicated by the small to moderate positive z value, there is not enough evidence to confidently claim superiority over a placebo [z = 1.48 (p = 0.14)].
Keywords: botulinum toxin; early injection; late injection; pain-related post-stroke spasticity; spasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Evaluating the functional outcomes of ultrasound-guided botulinum toxin type A injections using the Euro-musculus approach for upper limb spasticity treatment in post-stroke patients: an observational study.Eur J Phys Rehabil Med. 2018 Oct;54(5):738-744. doi: 10.23736/S1973-9087.18.05086-4. Epub 2018 Feb 7. Eur J Phys Rehabil Med. 2018. PMID: 29422486
-
The Complex Role of Botulinum Toxin in Enhancing Goal Achievement for Post-Stroke Patients.Toxins (Basel). 2024 Mar 31;16(4):172. doi: 10.3390/toxins16040172. Toxins (Basel). 2024. PMID: 38668597 Free PMC article.
-
Real-world analysis of botulinum toxin (BoNT) injections in post-stroke spasticity: Higher doses of BoNT and longer intervals in the early-start group.J Neurol Sci. 2021 Jun 15;425:117449. doi: 10.1016/j.jns.2021.117449. Epub 2021 Apr 17. J Neurol Sci. 2021. PMID: 33878656
-
Botulinum toxin as early intervention for spasticity after stroke or non-progressive brain lesion: A meta-analysis.J Neurol Sci. 2016 Dec 15;371:6-14. doi: 10.1016/j.jns.2016.10.005. Epub 2016 Oct 11. J Neurol Sci. 2016. PMID: 27871449 Review.
-
Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence.J Neural Transm (Vienna). 2009 Mar;116(3):319-31. doi: 10.1007/s00702-008-0175-8. Epub 2009 Jan 14. J Neural Transm (Vienna). 2009. PMID: 19142573 Review.
References
-
- World Stroke Organization World Stroke Organization Fact Sheet. 2022. [(accessed on 1 August 2024)]. Available online: https://www.world-stroke.org/assets/downloads/WSO_Global_Stroke_Fact_She....
-
- Biering-Soerensen B., Stevenson V., Bensmail D., Grabljevec K., Martínez Moreno M., Pucks-Faes E., Wissel J., Zampolini M. European Expert Consensus on Improving Patient Selection for the Management of Disabling Spasticity with Intrathecal Baclofen and/or Botulinum Toxin Type A. J. Rehabil. Med. 2022;54:jrm00241. doi: 10.2340/16501977-2877. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

