Alpha-Lipoic Acid Alleviates Lead-Induced Testicular Damage in Roosters by Reducing Oxidative Stress and Modulating Key Pathways
- PMID: 40423420
- PMCID: PMC12116080
- DOI: 10.3390/toxics13050341
Alpha-Lipoic Acid Alleviates Lead-Induced Testicular Damage in Roosters by Reducing Oxidative Stress and Modulating Key Pathways
Abstract
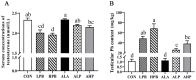
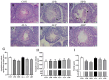
(1) Background: This study aimed to detect whether alpha-lipoic acid (ALA) supplementation could reduce lead (Pb)-induced testicular toxicity in roosters. (2) Methods: A total of 48 roosters, aged 20 weeks, were selected and randomly allocated to six treatment groups: basic diet (CON); CON + 150 mg/kg (CH3OO)2Pb (LPB); CON + 300 mg/kg (CH3OO)2Pb (HPB); CON + 300 mg/kg ALA (ALA); LPB + 300 mg/kg ALA (ALP); and HPB + 300 mg/kg ALA (AHP). (3) Results: The testicular Pb content was obviously higher in the LPB and HPB groups than in the CON group, while ALA supplementation reduced the testicular Pb content (p < 0.05). Roosters showed a significant increase in serum testosterone, sperm viability, sperm concentration, and testicular score in the AHP group compared with the HPB group. Pb exposure caused a remarkable increase in sperm abnormality and testicular malondialdehyde level, which were down-regulated by ALA supplementation (p < 0.05). RNA sequencing identified 227 differentially expressed genes (DEGs) between the HPB and CON groups and 220 DEGs between the HPB and AHP groups. (4) Conclusions: ALA supplementation mitigated Pb-induced testicular damage, suggesting its potential as a therapeutic agent for Pb toxicity in birds and potentially other species.
Keywords: Pb; alpha-lipoic acid; oxidative stress; rooster; testicular toxicity.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





References
-
- Godinez-Solis Y., Solis-Heredia M.J., Roa-Espitia A., Parra-Forero L.Y., Hernandez-Gonzalez E.O., Hernandez-Ochoa I., Quintanilla-Vega B. Low concentrations of lead decrease the sperm fertilization ability by altering the acrosome reaction in mice. Toxicol. Appl. Pharmacol. 2019;380:114694. doi: 10.1016/j.taap.2019.114694. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

