Influence of mutations at different distances from the active center on the activity and stability of laccase 13B22
- PMID: 40423903
- PMCID: PMC12116972
- DOI: 10.1186/s40643-025-00893-6
Influence of mutations at different distances from the active center on the activity and stability of laccase 13B22
Abstract
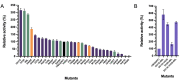
Laccases with high catalytic efficiency and high thermostability can drive a broader application scope. However, the structural distribution of key amino acids capable of significantly influencing the performance of laccases has not been explored in depth. Thirty laccase 13B22 mutants with changes in amino acids at distances of 5 Å (first shell), 5-8 Å (second shell), and 8-12 Å (third shell) from the active center were validated experimentally with 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) as substrate. Twelve of these mutants (first shell, 1; second shell, 4; third shell, 7) showed higher catalytic efficiency than the wild-type enzyme. Mutants D511E and I88L-D511E showed 5.36- and 10.58-fold increases in kcat/Km, respectively, with increases in optimal temperature of 15 °C and optimal pH from 7.0 to 8.0. Furthermore, both mutants exhibited greater thermostability compared to the wild-type, with increases of 3.33 °C and 5.06 °C in Tm and decreases of 0.39 J and 0.59 J in total structure energy, respectively. The D511E mutation resides in the third shell, while I88L is in the second shell, and their performance enhancements were attributed to alterations in the rigidity or flexibility of specific protein structural domains. Both mutants showed enhanced degradation efficiency for benzo[a]pyrene and zearalenone. These findings highlight the importance of the residues located far from the active center in the function of laccase (second shell and third shell), suggesting broader implications for enzyme optimization and biotechnological applications.
Keywords: Catalytic activity; Laccase; Mutant; Structural distance; Thermostability.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures




References
-
- Brodkin HR, Novak WR, Milne AC, D’Aquino JA, Karabacak NM, Goldberg IG, Agar JN, Payne MS, Petsko GA, Ondrechen MJ, Ringe D (2011) Evidence of the participation of remote residues in the catalytic activity of Co-type nitrile hydratase from Pseudomonas putida. Biochemistry 50(22):4923–4935 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

