Cell-based artificial platelet production: historical milestones, emerging trends, and future directions
- PMID: 40423909
- PMCID: PMC12116405
- DOI: 10.1007/s44313-025-00071-9
Cell-based artificial platelet production: historical milestones, emerging trends, and future directions
Abstract
Cell-based artificial platelet production has made remarkable progress over the past three decades, driven by the need for safe and stable platelet sources in the face of donor limitations and transfusion-related risks. This review provides a chronological overview of the evolution of in vitro platelet production from various cell sources (CD34+ hematopoietic stem cells, embryonic stem cells, induced pluripotent stem cells (iPSCs), and others) and highlights key advances in the field. We outline developments from the foundational experiments of the 1990s, through the introduction of iPSCs in the mid-2000s, to the adoption of three-dimensional culture and bioreactor technologies in the late 2010s and the emergence of clinical trials in the 2020s. In addition, we discuss future perspectives, including the role of advanced gene editing and scalable biomanufacturing technologies in accelerating clinical translation. This comprehensive review underscores the promise of artificial platelet production technologies for clinical applications and discusses the remaining challenges, such as scalability, cost-effectiveness, and regulatory hurdles. The recent completion of the first human clinical trials using iPSC-derived platelets marks a significant milestone, pointing to a future in which patient-specific or human leukocyte antigen-universal platelets may be transformed into transfusion medicine and regenerative therapies.
Keywords: Cell-based artificial platelets; Hematopoietic stem cells; Human pluripotent stem cells; Megakaryocytes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Seung Yeob Lee was the Assistant Editor of Blood Research while conducting this study. Editorial Board Member status has no bearing on editorial consideration.
Figures
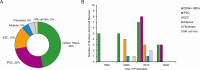
References
Publication types
Grants and funding
- RS-2023-KH140972, HX23C1706/Korean Cell-Based Artificial Blood Project funded by the Korean government (The Ministry of Science and ICT, The Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety
- RS-2023-KH140972, HX23C1706/Korean Cell-Based Artificial Blood Project funded by the Korean government (The Ministry of Science and ICT, The Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety
- RS-2023-KH140972, HX23C1706/Korean Cell-Based Artificial Blood Project funded by the Korean government (The Ministry of Science and ICT, The Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety
- RS-2023-KH140972, HX23C1706/Korean Cell-Based Artificial Blood Project funded by the Korean government (The Ministry of Science and ICT, The Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety
- RS-2023-KH140972, HX23C1706/Korean Cell-Based Artificial Blood Project funded by the Korean government (The Ministry of Science and ICT, The Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety
- RS-2023-KH140972, HX23C1706/Korean Cell-Based Artificial Blood Project funded by the Korean government (The Ministry of Science and ICT, The Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety
- RS-2023-KH140972, HX23C1706/Korean Cell-Based Artificial Blood Project funded by the Korean government (The Ministry of Science and ICT, The Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety
- RS-2023-KH140972, HX23C1706/Korean Cell-Based Artificial Blood Project funded by the Korean government (The Ministry of Science and ICT, The Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety
- NRF-2022R1C1C1011518/National Research Foundation
- NRF-2022R1C1C1011518/National Research Foundation
- NRF-2022R1C1C1011518/National Research Foundation
- NRF-2022R1I1A2070940/National Research Foundation
- NRF-2022R1C1C1011518/National Research Foundation
- RS-2023-00236157/Bio&Medical Technology Development Program of the NRF funded by the Korean government (MSIT)
- RS-2023-00236157/Bio&Medical Technology Development Program of the NRF funded by the Korean government (MSIT)
LinkOut - more resources
Full Text Sources
Miscellaneous

