Hydrocortisone and Risk Factors for Kidney Replacement Therapy in Septic Shock
- PMID: 40423970
- PMCID: PMC12117457
- DOI: 10.1001/jamanetworkopen.2025.12279
Hydrocortisone and Risk Factors for Kidney Replacement Therapy in Septic Shock
Abstract
Importance: Sepsis-associated acute kidney injury (SA-AKI) is a common and clinically important condition in patients who are critically ill. Dysregulated inflammation may contribute to it. Intravenous hydrocortisone may decrease the risk of SA-AKI progression.
Objective: To describe the associations of hydrocortisone use with the incidence and outcomes of requirement for kidney replacement therapy (KRT), as well as source of sepsis, mean arterial pressure (MAP), and MAP indexed to required vasopressor (norepinephrine equivalent [NEE]).
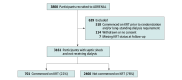
Design, setting, and participants: This cohort study was conducted as a post hoc analysis of the Adjunctive Corticosteroid Treatment in Critically Ill Patients with Septic Shock (ADRENAL) randomized clinical trial (RCT), a multicenter placebo-controlled RCT of hydrocortisone in patients with septic shock in 69 intensive care units in Australia, the United Kingdom, New Zealand, Saudi Arabia, and Denmark that recruited between 2013 and 2017. Participants were patients enrolled in the ADRENAL study with septic shock who did not require KRT in the 24 hours prior to randomization and who did not have a prior longstanding dialysis requirement. Data were analyzed between July and September 2024.
Exposures: Receipt of hydrocortisone (vs placebo), MAP at enrollment, vasopressor dose (NEE) and MAP:NEE ratio, source of sepsis, causative organism, bacteremia, and the use of nephrotoxic antimicrobials, vasopressin, or specific inotropes.
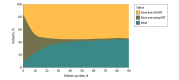
Main outcomes and measures: Outcomes of interest were KRT requirement and liberation from KRT, measured as days alive and free of KRT.
Results: A cohort of 3161 patients (median [IQR] age, 65 [53-74] years, 1921 [61%] male) was identified, including 1589 patients randomized to receive hydrocortisone and 1572 patients who received the placebo. Allocation to treatment with hydrocortisone was associated with a significantly reduced incidence of KRT requirement compared with placebo (329 patients [21%] vs 372 patients [24%]; odds ratio [OR], 0.84 [95% CI, 0.70 to 0.99]; P = .04). When controlled for factors associated with KRT requirement, randomization to hydrocortisone remained significantly associated with a reduced odds of new KRT requirement (OR, 0.79 [95% CI, 0.66 to 0.95]; P = .01). Among patients who started KRT following randomization, hydrocortisone was not associated with reduced days alive and free of KRT (mean difference, 1.28 [95% CI, -4.31 to 6.87] days; P = .65).
Conclusions and relevance: In this post hoc cohort study of patients with septic shock enrolled in a large RCT, intravenous hydrocortisone was associated with a reduced risk of new KRT requirement following randomization.
Conflict of interest statement
Figures
Comment in
References
-
- White KC, Serpa-Neto A, Hurford R, et al. ; Queensland Critical Care Research Network (QCCRN) . Sepsis-associated acute kidney injury in the intensive care unit: incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes—a multicenter, observational study. Intensive Care Med. 2023;49(9):1079-1089. doi: 10.1007/s00134-023-07138-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

