Phase 2 trial of cyclosporine-A, mycophenolate mofetil, and tocilizumab GVHD prophylaxis in cord blood transplantation
- PMID: 40423982
- PMCID: PMC12235316
- DOI: 10.1182/bloodadvances.2024014177
Phase 2 trial of cyclosporine-A, mycophenolate mofetil, and tocilizumab GVHD prophylaxis in cord blood transplantation
Abstract
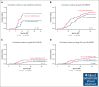
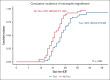
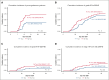
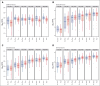
Double-unit cord blood transplantation (dCBT) has been associated with high rates of progression-free survival (PFS) in adults with hematologic malignancies but also with relatively high rates of acute graft-versus-host disease (aGVHD). We conducted a single-arm, phase 2 clinical trial that investigated the addition of tocilizumab, an interleukin-6 receptor blocker, to cyclosporine-A (CSA) and mycophenolate mofetil (MMF) for aGVHD prophylaxis after intermediate-intensity dCBT. A total of 45 patients (median age, 47 years; range, 27-60 years; 80% acute leukemia; median hematopoietic cell transplant-comorbidity index, 2) were enrolled from March 2018 to March 2021. Transplant outcomes were compared with 39 previous CSA and MMF dCBT controls with similar inclusion criteria. Tocilizumab recipients had less pre-engraftment syndrome (38%; 95% confidence interval [CI], 24-52 vs 72%; 95% CI, 54-84; P < .001) but inferior day 45 neutrophil engraftment (93%; median, 25.5 days vs 97%; median, 22 days; P = .009]. The primary end point of day 100 grade 2 to 4 aGVHD was no different between groups (71%; 95% CI, 55-82 with tocilizumab vs 82%; 95% CI, 65-91; P = .11). However, there was a trend toward a lower day 100 incidence of stage 1 to 4 lower gastrointestinal aGVHD with tocilizumab (16%; 95% CI, 7-28 vs 33%; 95% CI, 19-48; P = .059). There were no significant differences in the 3-year incidences of relapse, transplant-related mortality, PFS, or overall survival between the groups. Tocilizumab recipients exhibited a distinct pattern of gut microbiome disruption. In summary, tocilizumab-based GVHD prophylaxis delayed neutrophil recovery without a significant reduction in aGVHD and had no survival benefit after dCBT. Investigation of alternative strategies to prevent severe aGVHD after dCBT is warranted. This trial was registered at www.clinicaltrials.gov as #NCT03434730.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: I.P. reports receiving research funding from Merck; honoraria from PRECISIONheor; and serving on a data and safety monitoring board (DSMB) for ExCellThera. A.S. reports serving as a consultant on the scientific advisory board of ExCellThera. S.A.G. reports serving as a consultant for and receiving honoraria and research funding from Celgene and Novartis; serving as a consultant for and receiving research funding from Amgen, Actinuum, and Miltenyi Biotech; serving as a consultant for and receiving honoraria from Jazz Pharmaceuticals and Omeros; receiving research funding from Takeda; and serving as a consultant for Kite Pharma. B.G. reports receiving research funding from Actinium Pharmaceuticals and serving on the DSMB for Synthetic Biologics, Inc. M.A.P. reports receiving honoraria from Adicet, Allogene, AlloVir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Sanofi, Syncopation, VectivBio AG, and Vor Biopharma; serving on DSMBs for Cidara Therapeutics and Sellas Life Sciences; serving on on the scientific advisory board of NexImmune; reports ownership interests in NexImmune, Omeros, and OrcaBio; and reports institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. D.M.P. reports serving as a consultant for Kadmon/Sanofi Corporation, CareDx, Incyte, and Ceramedix, and receiving research funding from Incyte. B.C.S. reports receiving consulting fees from Hansa Biopharma and Gamida Cell. R.T. reports serving as a Blood and Marrow Transplant Clinical Trials Network medical monitor for Angiocrine Bioscience and Omeros. J.W.Y. reports owning equity in Merck, Pfizer, and Amgen. J.U.P. reports receiving research funding, intellectual property fees, and travel reimbursement from Seres Therapeutics and consulting fees from DaVolterra, CSL Behring, Crestone Inc, and from MaaT Pharma. He reports serving on an advisory board of and holding equity in Postbiotics Plus Research and Prodigy Biosciences. He further reports filing intellectual property applications related to the microbiome (reference numbers #62/843,849, #62/977,908, and #15/756,845). Memorial Sloan Kettering Cancer Center has financial interests relative to Seres Therapeutics. The remaining authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

