High-affinity detection of biotinylated endogenous neuroligin-1 at excitatory and inhibitory synapses using a tagged knock-in mouse
- PMID: 40424132
- PMCID: PMC12146770
- DOI: 10.1073/pnas.2411669122
High-affinity detection of biotinylated endogenous neuroligin-1 at excitatory and inhibitory synapses using a tagged knock-in mouse
Abstract
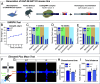
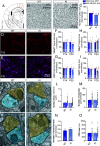
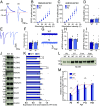
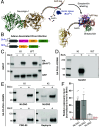
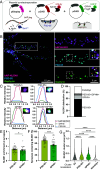
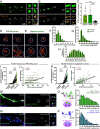
Neuroligins (NLGNs) are important cell adhesion molecules mediating trans-synaptic contacts between neurons. However, the high-yield biochemical isolation and visualization of endogenous NLGNs is hampered by the lack of efficient antibodies. Thus, to reveal their subcellular distribution, binding partners, and synaptic function, NLGNs were extensively manipulated using knock-down, knock-out, or overexpression approaches, leading to controversial results. As an alternative to the manipulation of NLGN expression level, we describe here the generation of a knock-in (KI) mouse strain in which native NLGN1 was N-terminally tagged with a small biotin acceptor peptide (bAP) that can be enzymatically biotinylated by the exogenous delivery of biotin ligase. After showing that KI mice exhibit normal behavior as well as similar synaptic number, ultrastructure, transmission properties, and protein expression levels when compared to wild type counterparts, we exploited the fact that biotinylated bAP-NLGN1 can be selectively isolated or visualized using high-affinity streptavidin conjugates. Using immunoblotting and immunofluorescence, we show that bAP-NLGN1 binds PSD-95 and gephyrin and populates both excitatory and inhibitory synapses, challenging the historical view that NLGN1 is exclusively localized at excitatory synapses. Using superresolution optical and electron microscopy, we further highlight that bAP-NLGN1 forms in the synaptic cleft a subset of nanodomains, which contain each a few NLGN1 dimers and whose number positively scales with the postsynapse size. Overall, our study not only provides an extensively characterized KI mouse model which will be available to the scientific community but also an unprecedented view of the nanoscale organization of endogenous NLGN1.
Keywords: biotinylation; cell adhesion molecule; knock-in mouse; neuroligin-1; synapse.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Poulopoulos A., et al. , Homodimerization and isoform-specific heterodimerization of neuroligins. Biochem. J. 446, 321–330 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

