Repetitive Transcranial Magnetic Stimulation targeted with MRI based neuro-navigation in major depressive episode: a double-blind, multicenter randomized controlled trial
- PMID: 40424301
- PMCID: PMC12111610
- DOI: 10.1371/journal.pone.0317597
Repetitive Transcranial Magnetic Stimulation targeted with MRI based neuro-navigation in major depressive episode: a double-blind, multicenter randomized controlled trial
Abstract
Context: High-frequency (HF) transcranial magnetic stimulation (rTMS) of the left dorsolateral prefrontal cortex (DLPFC) is widely used in Major Depressive Episode (MDE). Optimization of its efficacy with a neuro-navigation system has been proposed based on a small randomized controlled trial (RCT) supporting a large effect.
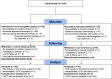
Method: This evaluator- and patient-blind, multicenter RCT assessed the superiority in terms of efficacy of 10 HF rTMS sessions of the left DLPFC targeted with MRI based neuro-navigation versus similar sessions targeted by the standard 5 cm technique. The study was conducted between January 2013 and April 2017, at 4 hospitals centers in France where both in- and out- patients with MDE were included. Randomization was computer-generated (1:1), with allocation concealment implemented within the e-CRF. The main outcome measure was the percentage of responders 44 days (D44) after the rTMS session. Secondary outcomes were percentage of remitters, Beck Depression Inventory and psychomotor retardation assessed with Salpêtrière retardation rating scale (SRRS) for depression at D14 and D44. The results are presented along with their 95% confidence intervals.
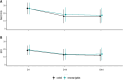
Results: 105 patients were randomized and 92 were evaluable with respectively 45 patients in the neuronavigation group and 47 in the standard group. A treatment response was observed for 14 (31.8%) of 44 patients analyzed in the intervention group, and for 16 (35.6%) of 45 patients analyzed in the control group with no statistical difference (relative risk 0.89; 95% confidence interval, [0.50;1.61]). No difference was evidenced for secondary outcomes at D44 whether it concerns remission at D44 (relative risk, 0.82; 95% CI, 0.36 to 1.88), or BDI results (difference in means, 0,01; 95% CI, -3.06 to 3.26), or SRRS results (difference in means, 0.11; 95% CI, -2.42 to 5.02). Similar results were observed at D14. Rates of adverse events were similar in both groups with 23 (47.9%) and 1 (2.1%) of adverse events and serious adverse events in the neuro-navigation group versus 20 (40.8%) and 0 (0%) in the standard group.
Discussion: This study failed to reproduce previous findings supporting the use of neuro-navigation system to optimize rTMS efficacy. Limitations of this study includes a small sample size and a number of rTMS sessions that may appear substandard in 2025.
Trial registration: NCT01677078.
Copyright: © 2025 Millet et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form available at http://www.icmje.org/coi_disclosure.pdf, which can be requested from the corresponding author. The authors declare that they have no conflicts of interest, except for Prof. Millet, who holds stocks in Syneika company. Dr Nauczyciel who is syneika CEO spouse worked on Prof. Millet's team and helped with data acquisition without fulfilling authorship criteria. These potential conflict of interest do not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Lefaucheur J-P, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al.. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin Neurophysiol. 2020;131(2):474–528. doi: 10.1016/j.clinph.2019.11.002 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous