Long lived liver-resident memory T cells of biased specificities for abundant sporozoite antigens drive malaria protection by radiation-attenuated sporozoite vaccination
- PMID: 40424562
- PMCID: PMC12143544
- DOI: 10.1371/journal.ppat.1012731
Long lived liver-resident memory T cells of biased specificities for abundant sporozoite antigens drive malaria protection by radiation-attenuated sporozoite vaccination
Abstract
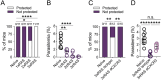
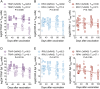
Vaccination with radiation-attenuated sporozoites (RAS) can provide highly effective protection against malaria in both humans and mice. To extend understanding of malaria immunity and inform the development of future vaccines, we studied the protective response elicited by this vaccine in C57BL/6 mice. We reveal that successive doses of Plasmodium berghei RAS favour the generation of liver CD8+ tissue-resident memory T cells (TRM cells) over circulating memory cells and markedly enhance their longevity. Importantly, RAS immunisation strongly skews the composition of the liver CD8+ TRM compartment towards cells specific for abundant sporozoite antigens, such as thrombospondin-related anonymous protein (TRAP) and circumsporozoite protein (CSP), which become major mediators of protection. The increased prevalence of sporozoite specificities is associated with limited intrahepatic attenuated parasite development and inhibition of naïve T cell responses to all parasite antigens, whether formerly encountered or not, in previously vaccinated mice. This leads to the exclusive expansion of effector T cells formed upon initial immunisation, ultimately reducing the diversity of the liver TRM pool later established. However, stronger responses to less abundant epitopes can be achieved with higher initial doses of RAS. These findings provide novel insights into the mechanisms governing malaria immunity induced by attenuated sporozoite vaccination and highlight the susceptibility of this vaccine to limitations imposed by strain-specific immunity associated with the abundant, yet highly variable sporozoite antigens CSP and TRAP.
Copyright: © 2025 de Menezes et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: DFR is a board member of iModulate Pty Ltd and RNAxis Pty Ltd. WRH is a board member of Avalia Immunotherapies Limited. MHL and IC are listed as inventors on patents relating to Clec9A antibodies. The authors have no additional financial interests. These competing interests will not alter adherence to PLOS policies on sharing data and materials.
Figures




Similar articles
-
Discriminating Protective from Nonprotective Plasmodium-Specific CD8+ T Cell Responses.J Immunol. 2016 May 15;196(10):4253-62. doi: 10.4049/jimmunol.1600155. Epub 2016 Apr 15. J Immunol. 2016. PMID: 27084099 Free PMC article.
-
Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites.PLoS Pathog. 2010 Jul 15;6(7):e1000998. doi: 10.1371/journal.ppat.1000998. PLoS Pathog. 2010. PMID: 20657824 Free PMC article.
-
Varying Immunizations With Plasmodium Radiation-Attenuated Sporozoites Alter Tissue-Specific CD8+ T Cell Dynamics.Front Immunol. 2018 May 28;9:1137. doi: 10.3389/fimmu.2018.01137. eCollection 2018. Front Immunol. 2018. PMID: 29892289 Free PMC article.
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
-
The role of intrahepatic lymphocytes in mediating protective immunity induced by attenuated Plasmodium berghei sporozoites.Immunol Rev. 2000 Apr;174:123-34. doi: 10.1034/j.1600-0528.2002.00013h.x. Immunol Rev. 2000. PMID: 10807512 Review.
References
-
- World malaria report 2023. Geneva: World Health Organization. 2023.
-
- Bloom DE. The Value of Vaccination. Springer New York. New York: Springer. 2011. 1–8.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

