Dynamics of heat shock protein 70 kDa in heat-shocked and hypoxic human endothelial cells
- PMID: 40425101
- PMCID: PMC12171560
- DOI: 10.1016/j.cstres.2025.100085
Dynamics of heat shock protein 70 kDa in heat-shocked and hypoxic human endothelial cells
Abstract
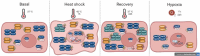
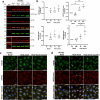
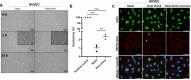
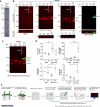
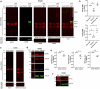
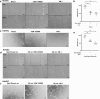
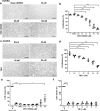
Heat shock proteins (HSPs) play crucial roles in human endothelial cell functions such as migration and angiogenesis. However, human HSP dynamics under stress conditions such as heat shock (HS) and hypoxia in human endothelial cells (ECs) are enigmatic, and the characteristics of HSPs in ECs after exposure to thermal stress and a low-oxygen environment are unknown. We hypothesized that ECs adapt to HS and hypoxia by modulating chaperome oligomerization and that HSP70 is a major determinant of the endothelial phenotype. HSP70 inhibition with VER-155008 or YM-1 in primary human endothelial cells decreases EC proliferation, migration, and angiogenesis at baseline and after heat shock recovery. We showed that vascular-independent HSC/P70 multimeric complexes in primary human veins (HUVEC) and coronary artery ECs (HCAEC) accumulate after HS and are decreased by hypoxia. HS recovery increases the number of HSP90 dimers, inducible HSP70, and HSP40 macromolecular complexes, whereas HSC70 returns to baseline. We demonstrated that the HS response and hypoxia regulate HSPs through a new layer of complexity, oligomerization, in addition to classical cochaperone/NEF interactions. The biphasic temporal oligomerization of molecular chaperones in the recovery phase provides a novel face of the heat shock response. In addition, shifts in the subcellular location and upregulation of HSP70 were also observed here. The decrease in HSP expression caused by hypoxia raises the possibility that decreased chaperone power contributes to the endothelial dysfunction found in atherosclerosis, thrombosis, and cancer. Together, these results show that HSP70 is pivotal to the healthy endothelial response in veins and coronary arteries, and we revealed human HSP dynamics in the vascular response to proteotoxic stress.
Keywords: Endothelial cell; HSP70; Heat-shock response; Hypoxia; Oligomerization.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declarations of interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Effects of Thermal Manipulation and Serotonin Modulation on Brain HSP70 and HSP90 Gene Expression in Late Embryogenesis of Broilers.Vet Med Sci. 2025 May;11(3):e70195. doi: 10.1002/vms3.70195. Vet Med Sci. 2025. PMID: 40172036 Free PMC article.
-
The Heat Shock Transcription Factor HsfA Plays a Role in Membrane Lipids Biosynthesis Connecting Thermotolerance and Unsaturated Fatty Acid Metabolism in Aspergillus fumigatus.Microbiol Spectr. 2023 Jun 15;11(3):e0162723. doi: 10.1128/spectrum.01627-23. Epub 2023 May 17. Microbiol Spectr. 2023. PMID: 37195179 Free PMC article.
-
Inflammatory mediators are perpetuated in macrophages resistant to apoptosis induced by hypoxia.Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):13903-8. doi: 10.1073/pnas.94.25.13903. Proc Natl Acad Sci U S A. 1997. PMID: 9391125 Free PMC article.
-
Hypoxia-inducible factor 1alpha and vascular endothelial growth factor in Glioblastoma Multiforme: a systematic review going beyond pathologic implications.Oncol Res. 2024 Jul 17;32(8):1239-1256. doi: 10.32604/or.2024.052130. eCollection 2024. Oncol Res. 2024. PMID: 39055895 Free PMC article.
-
The impact of stress in domestic animals: roles of heat shock proteins and acute-phase proteins.Vet Res Commun. 2025 Jul 17;49(5):258. doi: 10.1007/s11259-025-10802-z. Vet Res Commun. 2025. PMID: 40673978 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

