Topobexin targets the Topoisomerase II ATPase domain for beta isoform-selective inhibition and anthracycline cardioprotection
- PMID: 40425539
- PMCID: PMC12116762
- DOI: 10.1038/s41467-025-60167-9
Topobexin targets the Topoisomerase II ATPase domain for beta isoform-selective inhibition and anthracycline cardioprotection
Abstract
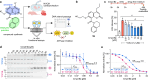
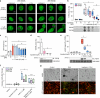
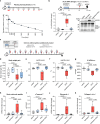
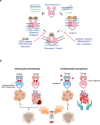
Topoisomerase II alpha and beta (TOP2A and TOP2B) isoenzymes perform essential and non-redundant cellular functions. Anthracyclines induce their potent anti-cancer effects primarily via TOP2A, but at the same time they induce a dose limiting cardiotoxicity through TOP2B. Here we describe the development of the obex class of TOP2 inhibitors that bind to a previously unidentified druggable pocket in the TOP2 ATPase domain to act as allosteric catalytic inhibitors by locking the ATPase domain conformation with the capability of isoform-selective inhibition. Through rational drug design we have developed topobexin, which interacts with residues that differ between TOP2A and TOP2B to provide inhibition that is both selective for TOP2B and superior to dexrazoxane. Topobexin is a potent protectant against chronic anthracycline cardiotoxicity in an animal model. This demonstration of TOP2 isoform-specific inhibition underscores the broader potential to improve drug specificity and minimize adverse effects in various medical treatments.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Obex compounds have been patented by Charles University and Mayo Clinic, with M.J.S., J.R., T.S., J.Kub, G.K., I.M., O.L., P.K., V.K., and M.S. listed as inventors (US patent application No. 63/534,074, 2023 and PCT application number PCT/US2024/043047). The remaining authors declare no competing interests.
Figures


References
-
- Tewey, K. M., Rowe, T. C., Yang, L., Halligan, B. D. & Liu, L. F. Adriamycin-Induced DNA Damage Mediated by Mammalian DNA Topoisomerase II. Science226, 466–468 (1984). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

