Physiologically relevant coculture model for oral microbial-host interactions
- PMID: 40425581
- PMCID: PMC12117109
- DOI: 10.1038/s41368-025-00365-9
Physiologically relevant coculture model for oral microbial-host interactions
Abstract
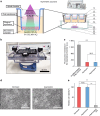
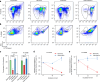
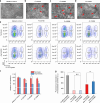
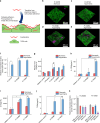
Understanding microbial-host interactions in the oral cavity is essential for elucidating oral disease pathogenesis and its systemic implications. In vitro bacteria-host cell coculture models have enabled fundamental studies to characterize bacterial infection and host responses in a reductionist yet reproducible manner. However, existing in vitro coculture models fail to establish conditions that are suitable for the growth of both mammalian cells and anaerobes, thereby hindering a comprehensive understanding of their interactions. Here, we present an asymmetric gas coculture system that simulates the oral microenvironment by maintaining distinct normoxic and anaerobic conditions for gingival epithelial cells and anaerobic bacteria, respectively. Using a key oral pathobiont, Fusobacterium nucleatum, as the primary test bed, we demonstrate that the system preserves bacterial viability and supports the integrity of telomerase-immortalized gingival keratinocytes. Compared to conventional models, this system enhanced bacterial invasion, elevated intracellular bacterial loads, and elicited more robust host pro-inflammatory responses, including increased secretion of CXCL10, IL-6, and IL-8. In addition, the model enabled precise evaluation of antibiotic efficacy against intracellular pathogens. Finally, we validate the ability of the asymmetric system to support the proliferation of a more oxygen-sensitive oral pathobiont, Porphyromonas gingivalis. These results underscore the utility of this coculture platform for studying oral microbial pathogenesis and screening therapeutics, offering a physiologically relevant approach to advance oral and systemic health research.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
Update of
-
Physiologically Relevant Coculture Model for Oral Microbial-Host Interactions.bioRxiv [Preprint]. 2025 Jan 14:2025.01.10.632380. doi: 10.1101/2025.01.10.632380. bioRxiv. 2025. Update in: Int J Oral Sci. 2025 May 27;17(1):42. doi: 10.1038/s41368-025-00365-9. PMID: 39868140 Free PMC article. Updated. Preprint.
References
-
- Attar, N. Microbial ecology: FISHing in the oral microbiota. Nat. Rev. Microbiol.14, 132–133 (2016). - PubMed
-
- Signat, B., Roques, C., Poulet, P. & Duffaut, D. Role of Fusobacterium nucleatum in periodontal health and disease. Curr. Issues Mol. Biol.13, 25–36 (2011). - PubMed
-
- Le Bars, P. et al. The oral cavity microbiota: between health, oral disease, and cancers of the aerodigestive tract. Can. J. Microbiol.63, 475–492 (2017). - PubMed
Publication types
MeSH terms
Grants and funding
- R03DE031329/U.S. Department of Health & Human Services | NIH | National Institute of Dental and Craniofacial Research (NIDCR)
- 2238972/National Science Foundation (NSF)
- R01DE030943/U.S. Department of Health & Human Services | NIH | National Institute of Dental and Craniofacial Research (NIDCR)
- R03 DE031329/DE/NIDCR NIH HHS/United States
- R01 DE030943/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources

