Ferroptosis-activating metabolite acrolein antagonizes necroptosis and anti-cancer therapeutics
- PMID: 40425585
- PMCID: PMC12116918
- DOI: 10.1038/s41467-025-60226-1
Ferroptosis-activating metabolite acrolein antagonizes necroptosis and anti-cancer therapeutics
Abstract
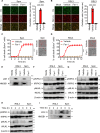
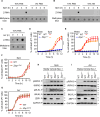
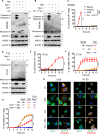
Dysregulated cell death leading to uncontrolled cell proliferation is a hallmark of cancer. Chemotherapy-induced cell death is critical for the success of cancer treatment but this process is impaired by metabolic byproducts. How these byproducts interfere with anti-cancer therapy is unclear. Here, we show that the metabolic byproduct acrolein derived from polyamines, tobacco smoke or fuel combustion, induces ferroptosis independently of ZBP1, while suppressing necroptosis in cancer cells by inhibiting the oligomerization of the necroptosis effector MLKL. Loss of the enzyme SAT1, which contributes to intracellular acrolein production, sensitizes cells to necroptosis. In mice, administration of an acrolein-trapping agent relieves necroptosis blockade and enhances the anti-tumor efficacy of the chemotherapeutic drug cyclophosphamide. Human patients with cancer coupled with a higher cell death activity but a lower expression of genes controlling polyamine metabolism exhibit improved survival. These findings highlight that the removal of metabolic byproducts improves the success of certain chemotherapies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Newton, K., Strasser, A., Kayagaki, N. & Dixit, V. M. Cell death. Cell187, 235–256 (2024). - PubMed
-
- Man, S. M. & Kanneganti, T. D. Innate immune sensing of cell death in disease and therapeutics. Nat. Cell Biol.26, 1420–1433 (2024). - PubMed
-
- Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature526, 660–665 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

