Inter- and intra-observer variability of software quantified bowel motility measurements of small bowel Crohn's disease: findings from the MOTILITY trial
- PMID: 40425892
- PMCID: PMC12116975
- DOI: 10.1186/s13244-025-01978-8
Inter- and intra-observer variability of software quantified bowel motility measurements of small bowel Crohn's disease: findings from the MOTILITY trial
Abstract
Objectives: Motility magnetic resonance imaging (mMRI) is a potential marker of disease activity of small bowel Crohn's disease (SBCD), but there is limited data on its reproducibility. We assessed inter- and intra-observer agreement of small bowel motility as part of a prospective multicentre trial investigating whether mMRI can predict longer-term response to biologic therapy in active, non-stricturing SB-CD (MOTILITY Trial).
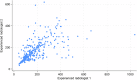
Methods: 297 segmental small bowel motility scores from 104 SBCD patients (mean age 38.9 years, 43 female) recruited to the MOTILITY trial were measured independently by two radiologists experienced in mMRI, using GIQuant software. Twenty-six datasets were re-read by both radiologists to test intra-observer variability after a washout period of at least 6 weeks. Five gastrointestinal radiologists inexperienced in mMRI derived 66 segmental motility scores from the same 30 randomly selected patients. Agreement was quantified using the intra-class correlation coefficient (ICC).
Results: There was moderate agreement for mMRI-derived segmental small bowel motility measurements for both mMRI-experienced and inexperienced radiologists (ICC 0.59 (95% CI: 0.51, 0.66) and 0.70 (95% CI: 0.61, 0.78), respectively). Agreement remained moderate to good, combining the experienced trial MRI reader measurements with those of the five inexperienced radiologists (ICC 0.69 (95% CI: 0.61, 0.78). Intra-observer agreement for the two mMRI experienced radiologists was (0.71 (95% CI: 0.44, 0.86) and 0.70 (95% CI: 0.44, 0.86)).
Conclusions: There is moderate to good interobserver agreement for mMRI measurements of segmental small bowel motility for both experienced and inexperienced radiologists.
Critical relevance statement: Study findings support the continuing clinical translation of motility MRI as a reproducible biomarker of disease activity and treatment response in Crohn's disease.
Key points: Motility MRI is a novel biomarker of small bowel Crohn's disease activity. Currently, limited data on intra- and inter-observer variability exists. Motility MRI shows moderate to good inter- and intra-observer agreement. Intraclass correlation was 0.59-0.71 for experienced and inexperienced radiologists. Motility MRI is reproducible, supporting its utility as a biomarker of disease activity.
Keywords: Biomarkers; Crohn disease; Gastrointestinal motility; Magnetic resonance imaging; Observer variation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The prospective trial was ethically approved (NHS West Midlands Research Ethics Committee: 17/WM/0106) and registered (ISRCTN14481560). The current study is a prespecified sub-study. Consent for publication: All study participants included provided informed, written consent as per the study protocol. Competing interests: S.T. is a consultant to AstraZeneca, has research grant support from Takeda, and is a shareholder in Motilent. G.B. is an employee of and shareholder in Motilent, is a consultant for Alimentiv, and owns the patent for P295276.US.02: system to characterise topology and morphology of fistulae from medical imaging data. The remaining authors declare no conflicts of interest.
Figures




References
-
- Turner D, Ricciuto A, Lewis A et al (2021) STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 160:1570–1583. 10.1053/j.gastro.2020.12.031 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

