Increasing the odds: antibody-mediated delivery of two distinct immunogenic T-cell epitopes with one antibody
- PMID: 40426019
- PMCID: PMC12118402
- DOI: 10.1080/2162402X.2025.2508050
Increasing the odds: antibody-mediated delivery of two distinct immunogenic T-cell epitopes with one antibody
Abstract
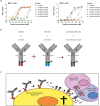
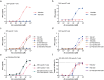
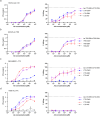
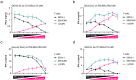
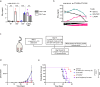
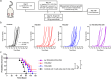
Antibody-epitope conjugates (AECs) proved to be a promising new therapeutic strategy to redirect virus-specific CD8+ T cells toward cancer cells by delivering T-cell epitopes. To be able to redirect a larger fraction of the virus-specific T-cell population, it is beneficial to deliver a broader selection of T-cell epitopes. We investigated two different methods to generate AECs with two distinct virus-specific T-cell epitopes fused to one antibody. Epitopes were either placed in a tandem-like fashion at the C-terminus of the AEC (t-AEC) or bispecific-AECs (bs-AECs) were generated via controlled Fab-arm exchange to generate bs-AECs with two identical antigen binding domains, but two distinct epitopes on each Fab-arm. Our study revealed that maintaining a free epitope terminus was required for efficient delivery of the virus-specific T-cell epitopes. Consequently, viral-epitope delivery using t-AECs was suboptimal as the concatenated epitopes were less effectively delivered to the target cells. However, well-defined bs-AECs containing both CMV and EBV epitopes were successfully generated and both in vitro and in vivo efficacy was evaluated. Our results demonstrate that bispecific-AECs can efficiently deliver EBV and CMV epitopes simultaneously to multiple cancer cell lines from different origins, thereby redirecting and activating two distinct populations of virus-specific T cells. Furthermore, our in vivo findings indicate that when both virus-specific T-cell populations are present and tumor cells express the proteases required for efficient epitope delivery, bs-AECs exhibit similar efficacy in reducing tumor burden compared to AECs. To conclude, our study demonstrates the feasibility of redirecting two groups of virus-specific T cells using a single antibody and highlights the potential of bs-AECs both in vitro and in vivo.
Keywords: Antibody-epitope conjugates (AECs); bispecific-AECs; immunotherapy; redirecting T-cells; tandem-AECs; virus-specific T-cells.
Conflict of interest statement
BB, JS, and LG are (former) employees at Genmab BV and have ownership interests (including stocks, warrants, patents, etc.).
Figures
References
-
- Jung K, Son MJ, Lee SY, Kim JA, Ko DH, Yoo S, Kim C-H, Kim Y-S.. Antibody-mediated delivery of a viral Mhc-I epitope into the cytosol of target tumor cells repurposes virus-specific Cd8(+) T cells for cancer immunotherapy. Mol Cancer. 2022;21(1):102. doi: 10.1186/s12943-022-01574-0. Epub 2022/04/24. - DOI - PMC - PubMed
-
- Sefrin JP, Hillringhaus L, Mundigl O, Mann K, Ziegler-Landesberger D, Seul H, Tabares G, Knoblauch D, Leinenbach A, Friligou I, et al. Sensitization of tumors for attack by virus-specific Cd8+ T-Cells through antibody-mediated delivery of immunogenic T-Cell Epitopes. Front Immunol. 2019;10:1962. doi: 10.3389/fimmu.2019.01962. Epub 2019/09/27. - DOI - PMC - PubMed
-
- van der Wulp W, Gram AM, Bleijlevens B, Hagedoorn RS, Araman C, Kim RQ, Drijfhout JW, Parren PWHI, Hibbert RG, Hoeben RC, et al. Comparison of methods generating antibody-epitope conjugates for targeting cancer with virus-specific T cells. Front Immunol. 2023;14:1183914. doi: 10.3389/fimmu.2023.1183914. Epub 2023/06/01. - DOI - PMC - PubMed
-
- Millar DG, Ramjiawan RR, Kawaguchi K, Gupta N, Chen J, Zhang S, Nojiri T, Ho WW, Aoki S, Jung K, et al. Antibody-mediated delivery of viral epitopes to tumors harnesses Cmv-specific T cells for cancer therapy. Nat Biotechnol. 2020;38(4):420–13. doi: 10.1038/s41587-019-0404-8. Epub 2020/02/12. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
