Development and validation of a preeclampsia prediction model for the first and second trimester pregnancy based on medical history
- PMID: 40426100
- PMCID: PMC12107935
- DOI: 10.1186/s12884-025-07733-7
Development and validation of a preeclampsia prediction model for the first and second trimester pregnancy based on medical history
Abstract
Objective: The study aimed to identify the risk factors of preeclampsia (PE) and establish a novel prediction model.
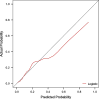
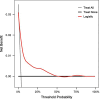
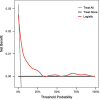
Study design: A retrospective, single-center analysis was conducted using clinical data from 5099 pregnant women who gave birth at Peking University People's Hospital between June 2015 and December 2020 who had placental growth factor (PIGF) levels records at 13-20 + 6 gestation weeks. The participants were randomly divided into a training set (70%, n = 3569) and a validation set (30%, n = 1030), between which the consistency was checked, and the analysis was performed according to whether PE occurred during pregnancy. Factors with univariate logistic analysis outcome of p < 0.2 were incorporated into the multivariate logistic regression analysis model, then variable selection by stepwise regression with AIC as the criterion was executed to finally identify the variables used for modeling. The model's discriminative ability was assessed using the receiver operating characteristic (ROC) curve, and its calibration was evaluated through calibration curves and Hosmer-Lemesow test. In addition, decision curve analysis (DCA) was used for clinical net benefit appraisal.
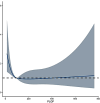
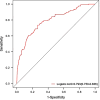
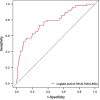
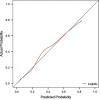
Results: Logistic regression analysis identified nine risk factors for PE, including: maternal age (OR = 1.072, 95%CI = 1.025-1.120), parity(OR = 0.718,95%CI = 0.470-1.060), pre-pregnancy BMI (OR = 2.842,95%CI = 1.957-4.106), family hypertension history (OR = 3.604,95%CI = 2.433-5.264), pregestational diabetes mellitus(PGDM) (OR = 8.399, 95%CI = 4.138-15.883), pregnancy complicating nephropathy (OR = 7.931, 95% CI = 2.584-20.258),pregnancy complicating immune system disorders (OR = 3.134, 95% CI = 1.624-5.525), mean arterial pressure(MAP) at 11-13 + 6 gestational weeks (OR = 1.098, 95% CI = 1.078-1.119) and PIGF (OR = 0.647, 95% CI = 0.448-0.927) at 13-20 + 6 gestational weeks (P < 0.05). The restricted spline regression analysis (RCS) analysis results showed that PIGF and the risk of PE presented an approximately "L-shaped" relationship, with the risk of PE rising sharply with the decrease of PIGF when PIGF < 90 pg/ml, and little change with the increase of PIGF when PIGF > 90 pg/ml. A risk prediction model for PE during the first and second trimester was constructed based on the above selected 11 factors. The area under the ROC curve (AUC) for the model was 0.781(95%CI = 0.709-0.853), and the sensitivity and specificity at the optimal cut-off value (threshold probability) were 0.571 and 0.879 respectively. Chi-square of 9.616 and P value of 0.293 from Hosmer-Lemeshow test indicated that the model was well calibrated. Finally, the model showed good clinical net benefits in the threshold range of 0.03-0.3.
Conclusion: The incidence of PE was associated with maternal age, pre-pregnancy weight and BMI, family hypertension history, PGDM, pregnancy complicating nephropathy, gestational complicating immune system disorders, blood pressure (systolic, diastolic, mean arterial pressure) at 11-13 + 6 gestational weeks, and PIGF at 13-20 + 6 gestational weeks. When PIGF < 90 pg/ml at 13-20 + 6 gestational week, the risk of PE increased significantly with the reduction of PIGF. The nomogram based on the above results was simpler and more practical in clinical application for PE predicting during the first and second trimester, and may provide an important reference for doctors and patients.
Keywords: Medical history; Multivariate logistic regression; Nomogram; Preeclampsia prediction model; The first and second trimester.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This retrospective study was approved by the Ethics Committee of the Peking University People’s Hospital (Approval NO.: 2024PHB298-001) and was conducted in accordance with the regulation of Measures for the Ethical Review of Life Science and Medical Research Involving Humans issued in 2023 in China and the Helsinki Declaration. Informed Consent to participate was waived by the Ethics Committee due to the study characteristics. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Macedo TCC, Montagna E, Trevisan CM, Zaia V, de Oliveira R, Barbosa CP, Laganà AS, Bianco B. Prevalence of preeclampsia and eclampsia in adolescent pregnancy: A systematic review and meta-analysis of 291,247 adolescents worldwide since 1969. Eur J Obstet Gynecol Reprod Biol. 2020;248:177–86. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical