Safety and efficacy of rebamipide compared to artificial tears for the treatment of dry eye: a systematic review and meta-analysis
- PMID: 40426110
- PMCID: PMC12107901
- DOI: 10.1186/s12886-025-04146-0
Safety and efficacy of rebamipide compared to artificial tears for the treatment of dry eye: a systematic review and meta-analysis
Abstract
Background: Rebamipide (RBM) is a novel drug that increases mucin secretion on the ocular surface. Nevertheless, the therapeutic efficacy of topical RBM for dry eye disease (DED) treatment remains inconclusive. Accordingly, we conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) investigating the effectiveness of RBM for DED treatment.
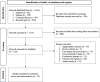
Methods: We searched the PubMed, Embase, and Cochrane Library databases for eligible RCTs. The primary outcome was posttreatment changes in tear breakup time (TBUT). We also assessed changes in the values of Schirmer's test (Sch), corneal fluorescein staining scores, and DED-related symptom scores.
Results: We retrieved 12 RCTs with 1368 eyes published during 2012-2023. The results demonstrated that compared with artificial tears, 2% RBM significantly increased the TBUT [standard mean difference (SMD) = 1.42, 95% confidence interval (CI) = 0.20 to 2.64]. Moreover, 2% RBM led to more significant improvements in overall DED-related symptom scores than did artificial tears (SMD = - 1.61, 95% CI = - 2.61 to - 0.61). The differences in the adverse events between the 2% RBM and artificial tears groups were nonsignificant (SMD = 1.23, 95% CI = 0.62 to 2.44).
Conclusion: Topical RBM ophthalmic suspension is a safe and effective treatment for DED patients. Compared to artificial tears, 2% RBM improved TBUT and subjective symptoms of DED. It may be considered as the first-line treatment option for short- TBUT dry eye patients.
Keywords: Dry eye disease; Meta-analysis; Rebamipide; Systematic review; Tear breakup time.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval is not required as our study will not include confidential participant data and intervention. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



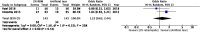
References
-
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007(2):75–92. 10.1016/s1542-0124(12)70081-2. PMID: 17508116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

