Pulmonary hazards of nanoplastic particles: a study using polystyrene in in vitro models of the alveolar and bronchial epithelium
- PMID: 40426130
- PMCID: PMC12117733
- DOI: 10.1186/s12951-025-03419-6
Pulmonary hazards of nanoplastic particles: a study using polystyrene in in vitro models of the alveolar and bronchial epithelium
Abstract
Background: Nanoplastics (NPs) are released into the environment through the degradation of plastic objects, leading to human exposure. Due to their small size, concerns have been raised about the potential hazards to the respiratory tract, as ultrafine and nanoparticles are known to penetrate till the alveolar regions of the lungs, potentially impairing their functions. Thus, in the present study, we used model polystyrene nanoparticles doped with the fluorescent metal europium (PS-Eu) to enhance the understanding of NPs hazard and investigate adverse outcomes associated with exposure in human lungs using alveolar (A549) and bronchial (Calu-3) cell models grown in 2D and 3D submerged conditions or quasi air-liquid interface (ALI) conditions (3D).
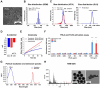
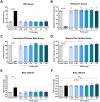
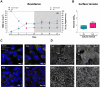
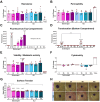
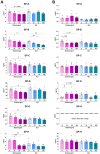
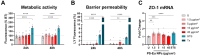
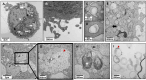
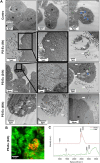
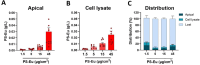
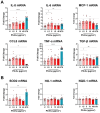
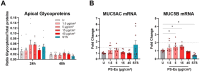
Results: Briefly, after in-dept physicochemical characterization of the particles, we assessed their impact on ROS production, cell viability (AlamarBlue and lactate dehydrogenase assays) and barrier integrity (lucifer yellow assay and TEER measurement), finding no negative effects in either model. However, in alveolar cells, particles increased acidic organelle activity. Transmission electron microscopy and Raman microscopy showed, in both models, a dose- and cell-dependent particle uptake with PS-Eu accumulating in numerous and large endo-lysosomes, which, in transwells-grown A549 cells, often contained also lamellar bodies (LBs), organelles involved in surfactants storage and secretion. After extensively quantifying surfactant proteins (SP) in the pellet and supernatant fractions of treated A549 cells, we observed a significant reduction in several members of this family, including surfactant protein B, which is crucial for lamellar body formation and surface tension regulation in the lungs. In quasi-ALI Calu-3 cultures instead, PS-Eu significantly upregulated interleukin 6 (IL-6) and increased transforming growth factor beta β (TGF-β), zonula occludens 1 (ZO-1), and mucin (MUC) 5B mRNA expressions causing a moderate proinflammatory response.
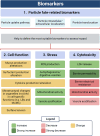
Conclusion: Our results show that PS-Eu exposure does not induce acute cytotoxicity in these models, but affects cell-specific functions like surfactant, mucin, and cytokine production. This underscores the limitations of relying solely on standard cytotoxicity tests for particle hazard assessment and highlights the importance of investigating cell function-specific signaling pathways. To support researchers in hazard assessment, we propose specific classes of biomarkers to test in in vitro lung models.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv [Internet]. 2017;3(7):e1700782. Available from: https://www.science.org/doi/abs/10.1126/sciadv.1700782 - PMC - PubMed
-
- Siddiqui SA, Singh S, Bahmid NA, Shyu DJH, Domínguez R, Lorenzo JM, et al. Polystyrene microplastic particles in the food chain: characteristics and toxicity. A review. Science of the Total Environment. Volume 892. Elsevier B.V.; 2023. - PubMed
-
- Kik K, Bukowska B, Sicińska P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environmental Pollution [Internet]. 2020;262:114297. Available from: https://www.sciencedirect.com/science/article/pii/S026974911933595X - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

