Real-world treatment patterns and outcomes for patients with non-metastatic non-small cell lung cancer: retrospective analyses in Canada, England, and Germany
- PMID: 40426148
- PMCID: PMC12107783
- DOI: 10.1186/s12890-025-03715-9
Real-world treatment patterns and outcomes for patients with non-metastatic non-small cell lung cancer: retrospective analyses in Canada, England, and Germany
Abstract
Background: Recent therapeutic advancements for non-metastatic non-small cell lung cancer (NSCLC) have increased the need for real-world baselines against which future changes in patient management and clinical outcomes can be compared.
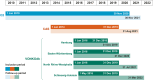
Methods: Data on patient characteristics, initial treatment, and overall survival (OS) were derived from adult patients diagnosed with stage I-IIIC NSCLC (2010-2020) in a regional Canadian database (Oncology Outcomes [O2]), an English national registry (Cancer Analysis System [CAS]), and four regional German registries (VONKOdb) and retrospectively analyzed separately using analogous methodology.
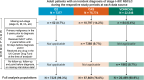
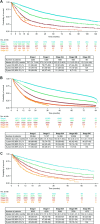
Results: Data from 85,433 patients were analyzed. Stage at diagnosis varied, with proportions with stage I NSCLC ranging from 30.9% (VONKOdb) to 44.2% (O2) and with stage III disease from 36.9% (O2) to 48.5% (VONKOdb). Across the data sources, proportions receiving surgery ± other treatments were similar for stages I and II, but decreased through stages IIIA, IIIB, and IIIC (range, 24.7-42.7%, 4.6-21.8%, and 0.9-7.5%, respectively). Overall, 70.3-85.2% of patients received active treatment for NSCLC, with a trend toward lower proportions among those with stage III disease. Reached median OS tended to be longest in patients with resected stage I/II NSCLC (range, 28.8-128.0 months) and shortest in patients with stage IIIB/IIIC disease treated with systemic anticancer therapy (SACT) alone, radiotherapy alone, or SACT + palliative radiotherapy (range, 4.8-21.2 months).
Conclusions: These data provide insights into treatment pathways and survival outcomes before the widespread use of immunotherapy-based and targeted therapies and will serve as an important baseline for future evaluations of emerging treatments for patients with non-metastatic NSCLC.
Keywords: Database; I-O Optimise; Real-world evidence; Registries; Survival; Treatment patterns.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Each data source–specific study was conducted in accordance with the relevant Good Epidemiological Practice guidelines (the International Society for Pharmacoepidemiology Guidelines for Good Pharmacoepidemiology Practices and/or the German guidelines and recommendations for ensuring Good Epidemiological Practice [68, 69]) and the ethical principles that have their origin in the Declaration of Helsinki. Respective applicable laws and regulatory requirements in Canada, England, and Germany were followed. The O2 protocol received approval by the Health Research Ethics Board of Alberta. The CAS and VONKOdb protocols did not require ethical approval; however, the VONKOdb data extraction and analyses were approved by the Ethics Committee of the University of Lübeck (#20–483). Individual patient data were analyzed by authorized staff at each data source, with only aggregated data released to the wider study teams. Given the country-specific rules described above, and since these were non-interventional, retrospective observational studies using pseudo-anonymized patient data, informed consent was not required. Consent for publication: Not applicable. Competing interests: The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: AG reports funding and medical writing support from Bristol Myers Squibb (BMS); consulting fees from AstraZeneca, BMS, MSD, and Roche; honoraria from AstraZeneca; support for attending meetings from Roche; and research funding (institution) from AstraZeneca. MJD reports employment by BMS at the time of these analyses, and stock or stock options in BMS. CR reports no conflicts of interest. HB reports funding and medical writing support from BMS. PQD reports no conflicts of interest. GE, SL, and LV report employment by BMS; SL and LV also report stock or stock options in BMS. VMS, ER, CL, and MJS report employment by IQVIA, a contract research organization contracted by the study sponsor, BMS. AK reports funding and medical writing support from BMS; and support for attending meetings from BMS. AW reports funding and medical writing support from BMS; and support for attending meetings from BMS. WYC reports no conflicts of interest.
Figures
References
-
- International Agency for Research on Cancer. GLOBOCAN 2022: Europe fact sheets. Available from: https://gco.iarc.who.int/media/globocan/factsheets/populations/908-europ.... Accessed 27 Jan 2025.
-
- International Agency for Research on Cancer. GLOBOCAN 2022: North America fact sheets. Available from: https://gco.iarc.who.int/media/globocan/factsheets/populations/905-north.... Accessed 27 Jan 2025.
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. - PubMed
-
- National Cancer Institute: Surveillance, Epidemiology, and End Results (SEER) Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed 27 Jan 2025.
-
- Sørensen JB, Horvat P, Rosenlund M, Kejs AM, Patel D, Juarez-Garcia A, Lacoin L, Daumont MJ, Penrod JR, O’Donnell JC, et al. Initial treatment and survival in Danish patients diagnosed with non-small-cell lung cancer (2005–2015): SCAN-LEAF study. Future Oncol. 2022;18(2):205–14. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

